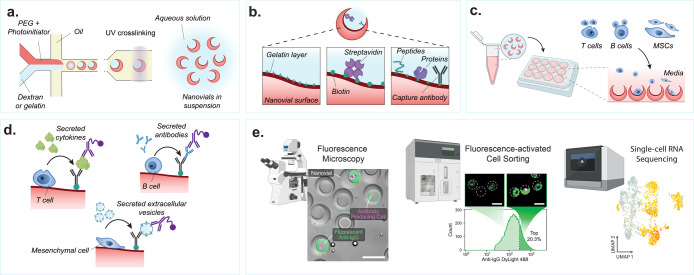
Figure 3.
Nanovial fabrication and experimental workflow. (a) An aqueous phase consisting of reactive PEG precursor and photoinitiator is coflowed with a second aqueous phase consisting of gelatin or dextran solution in a microfluidics droplet generator resulting in uniform monodispersed aqueous two-phase water-in-oil droplets. The phase-separated droplets are exposed to UV light downstream to polymerize the PEG phase. The dextran or gelatin sacrificial phase is removed during washing steps resulting in an open cavity and final crescent-shaped cross-sectional morphology. (b) If fabricated with gelatin, the nanovials will have a localized gelatin layer at the cavity surface. The gelatin or PEG surface can be functionalized with biotin and streptavidin moieties to attach peptides, proteins, or antibodies to localize cells and their secretions to individual nanovials. (c) Various cell types with a wide diversity of secreted products can be loaded onto and analyzed on nanovials. Cells are loaded onto nanovials in tubes or well plates in bulk, and unbound cells can be filtered out. (d) Fluorescent and/or oligo-barcode labeled detection antibodies are incubated with cells on nanovials to detect their secretions. (e) Single-cell secretion analysis is performed with microscopy, FACS, and/or single-cell sequencing techniques. Scale bar is 100 μm. [First two images reproduced from de Rutte, J., et al. Suspendable Hydrogel Nanovials for Massively Parallel Single-Cell Functional Analysis and Sorting. ACS Nano2022, 16 (5), 7242–7257 (ref (11)) Copyright 2022 American Chemical Society. Last image reprinted with permission from Macmillan Publishers Ltd.: Nature, Udani, S., et al. Nat. Nanotechnol. (ref (9)) Copyright 2023.]