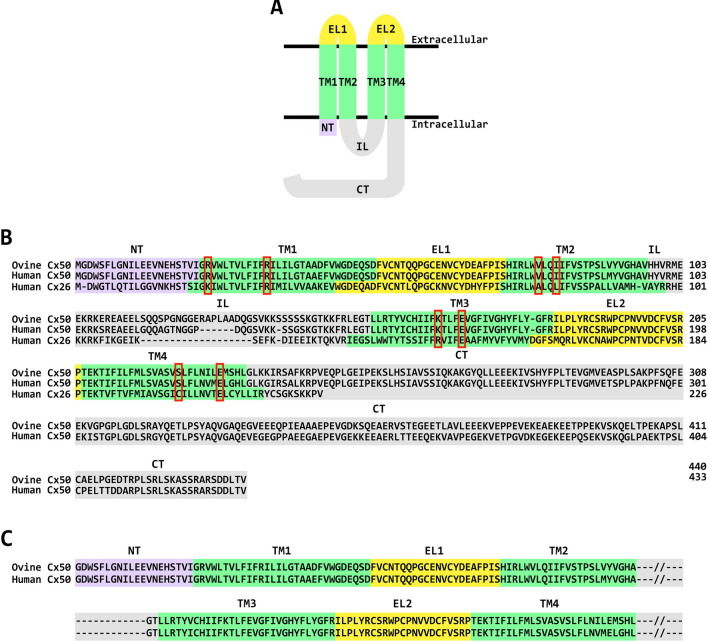
Fig. 1.
Connexin membrane topology and sequence conservation. A Diagram illustrating the membrane topology of a connexin monomer, indicating that it contains an amino terminus (NT, shown in mauve), four transmembrane domains (TM1–TM4, shown in green), two extracellular loops (EL1 and EL2, shown in yellow), an intracellular loop (IL, shown in gray), and a cytoplasmic carboxyl terminus (CT, shown in gray). B Sequence alignment of ovine and human Cx50 showing the high degree of amino acid sequence conservation between the two species, and of human Cx26. Amino acids have been assigned to specific protein domains in Cx50 according to the data reported in Fig. 4 by Myers et al. [6], and in Cx26 according to the data reported in Supplementary Fig. 3 by Maeda et al. [4]. The different domains have been enclosed by rectangles with colors that match those of the different domains in the diagram of the connexin monomer shown in A. The conserved amino acids involved in the formation of the intracellular pocket in Cx26 and Cx50 are indicated by the red boxes. C Sequence alignment of ovine and human Cx50 showing the amino acids included in our simulations based on the structure of the connexin channel reported by Myers et al. [6]. The intracellular loop and carboxyl terminus are not included, because they are not structured