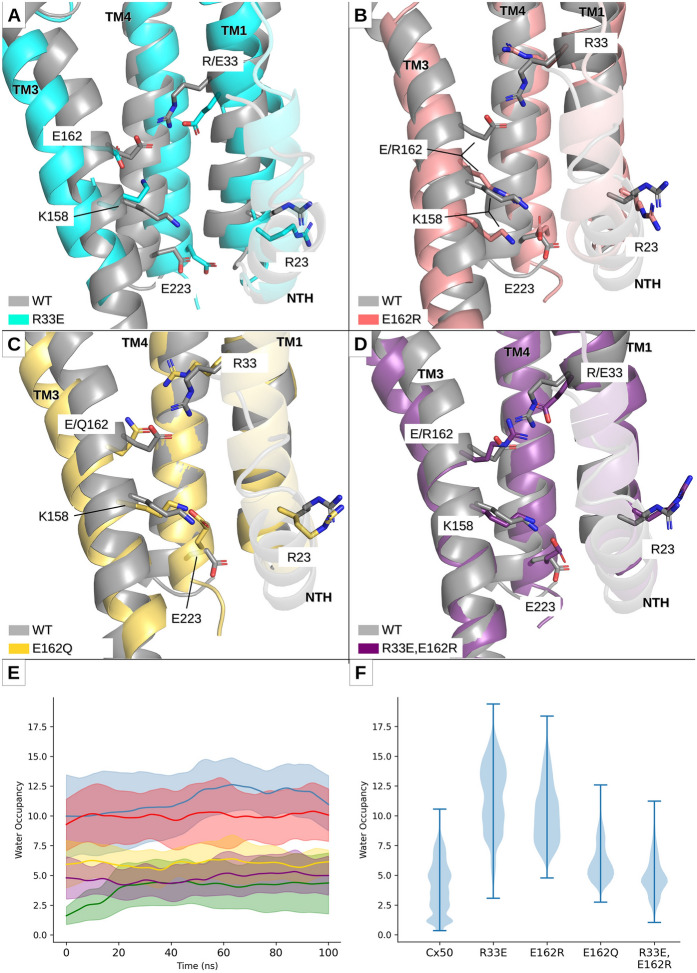
Fig. 3.
Comparison of the molecular environment around the intracellular pocket and of the distribution of water molecules between wild-type and mutant Cx50 protomers. A‒D The regions of the wild-type and mutant Cx50 protomers enclosing the majority of the volume of the intracellular pocket are shown in ribbon representation. The region of the wild-type protomer (shown in gray) has been superimposed upon the regions of the Cx50R33E (A), Cx50E162R (B), Cx50E162Q (C) and Cx50R33E,E162R (D) protomers to better illustrate the changes in tilting and twisting of their transmembrane domains compared with wild type. Simulations of wild-type Cx50 hemichannels show the salt-bridge interaction between amino acid residues R33 and E162 (R33-E162) within each protomer, and the absence of a salt-bridge interaction between amino acid residues R23 and E223. The ionic interaction between amino acid residues at positions 33 and 162 is lost in the single mutants, Cx50R33E, Cx50E162R and Cx50E162Q. In the Cx50R33E protomer, E162 can interact with K158 (A). In the wild-type Cx50, Cx50E162R, Cx50E162Q and Cx50R33E,E162R protomers, K158 can interact with E223 (B–D). This interaction does not occur in all six protomers, except in the case of the double charge substitution mutant hemichannels. In the protomer from the double substitution mutant, Cx50R33E,E162R, the mutated residues, E33 and R162, can form a salt-bridge interaction (D). All structures shown were extracted from the last frame of the corresponding simulation. E Graph shows the time-course of the number of water molecules inside the intracellular pocket during the 100-ns simulation in each system. Data are presented as the average (line) ± standard deviation (shade) of the 3 100-ns simulations performed for each system. F Violin plot shows the distribution of the number of water molecules inside the intracellular pocket. The data correspond to all frames from all three replicas for each system