FIGURE 3.
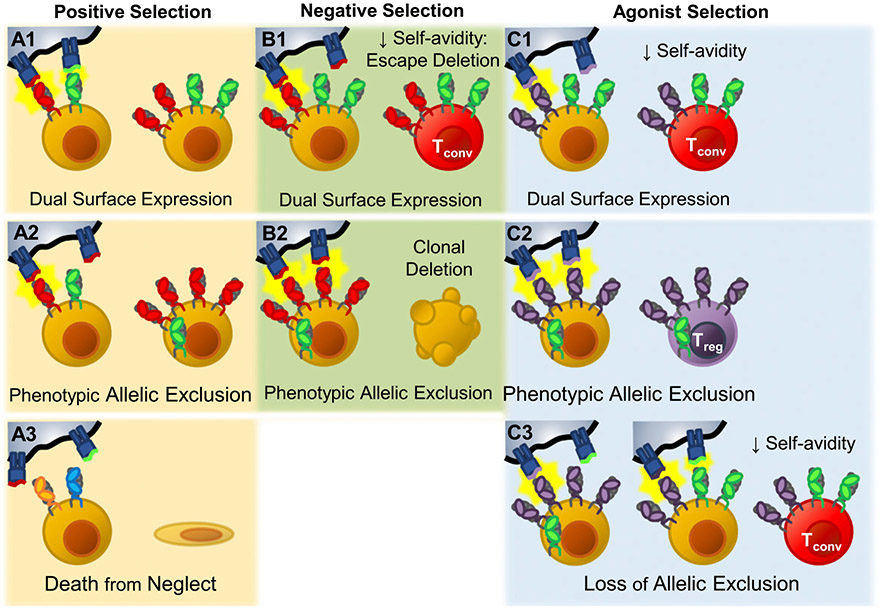
Effects of dual TCR expression on thymic selection. During positive selection, dual TCR–expressing thymocytes have three potential outcomes. (A1) Both TCRs are retained on the cell surface (Dual TCR T cell). (A2) Only one of the two TCRs is retained. The excluded TCR is preferentially internalized and degraded, leaving the other TCR to populate the cell surface, establishing phenotypic allelic exclusion. Phenotypic allelic exclusion is likely plastic; thus, these cells retain the potential to recognize both specificities. (A3) Neither TCR induces positive selection, and the cell dies from neglect. (B1) During negative selection, thymocytes that express two surface TCRs may experience decreased overall avidity to self-peptide–MHC relative to sole expression of the self-reactive TCR. In this situation dual TCR expression has the potential to allow the strongly self-reactive TCRs to escape clonal deletion. (B2) If phenotypic allelic exclusion has been established and the self-reactive TCR dominates the cell surface, avidity would likely be similar to sole expression of the self-reactive TCR, and the cell would undergo clonal deletion normally. Agonist selection of Treg cells takes place in the medulla where thymocytes are tested against new self-peptide–MHC combinations. (C1) If the thymocyte enters the medulla expressing two TCR specificities on its cell surface, it is possible that the resulting decrease in avidity could limit agonist-mediated commitment to the Treg cell lineage. (C2) If the phenotypic allelic exclusion established in the cortex is maintained in the medulla, agonist selection would be expected to function normally, and Treg cell–biasing TCRs would drive Treg cell commitment. (C3) However, if encountering new self-peptide–MHC combinations alters phenotypic allelic exclusion, it is possible that self-avidity could be reduced, effectively rerouting Treg cell–biasing TCRs into conventional T cell lineages.