Fig. 2.
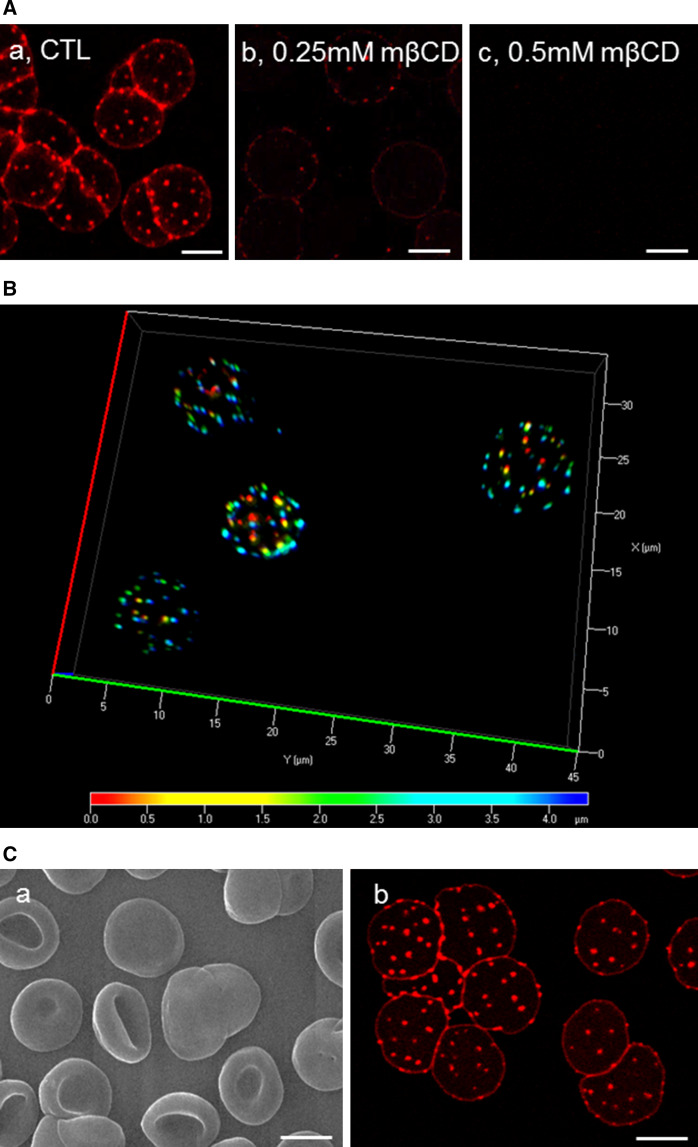
Theta* reveals numerous cholesterol submicrometric membrane domains on RBCs partially spread onto poly-l-lysine coverslips. A Submicrometric domains are numerous and cholesterol-dependent on living spread RBCs. Freshly isolated RBCs were either kept untreated (CTL; a) or cholesterol-depleted by 0.25 mM (b ~15 % cholesterol depletion, see Fig. 1C) or 0.5 mM (c ~25 % cholesterol depletion) mβCD at 37 °C, labeled in suspension with theta* in the continuous presence of mβCD, washed, attached-spread onto poly-l-lysine-coated coverslips and directly visualized by confocal microscopy at 20 °C. B Cholesterol submicrometric domains are present on both sides of living spread RBCs. Fresh RBCs were labeled and spread as at A and observed with wide-field fluorescence microscope. 3D-deconvolution and depth pseudo-coloration were then applied to visualize domain position in 3D (red corresponds to poly-l-lysine-free side, blue to poly-l-lysine-attached side). For rotating view, see Fig. S2. C Scanning electron and confocal microscopy of theta*-labeled, glutaraldehyde-fixed RBCs. RBCs were labeled and spread as above, then fixed with 0.5 % glutaraldehyde and processed either for scanning electron microscopy (a) or confocal microscopy (b). Notice that theta* labeling does not ultrastructurally alter the smooth RBC plasma membrane (a) and that glutaraldehyde fixation preserves theta* labeling of cholesterol submicrometric domains (b). All scale bars 5 µm