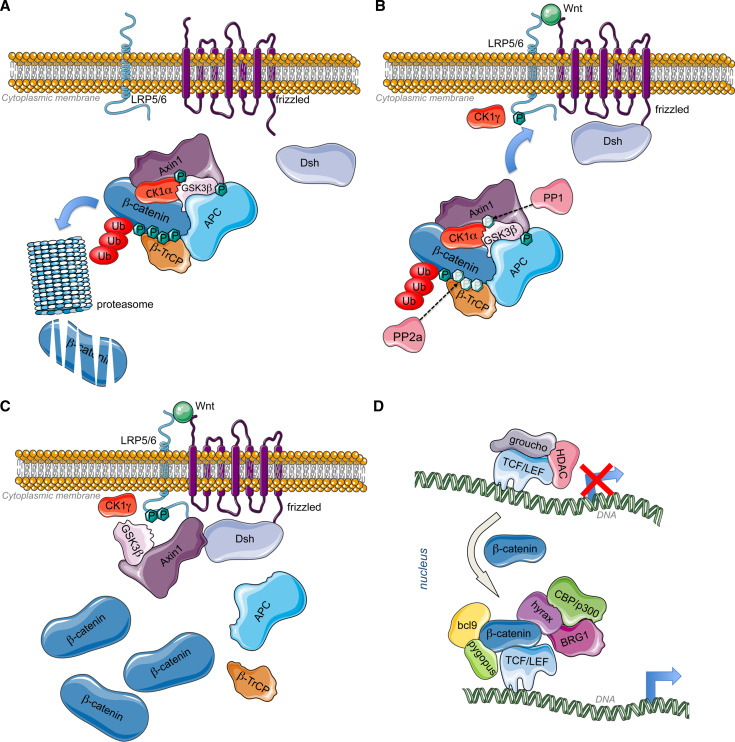
Fig. 1.
a Inactive Wnt pathway: Without Wnt ligands, CK1α phosphorylates axin for the binding of GSK3β that in turn phosphorylates APC. The scaffold formed by axin and APC allow the phosphorylation of β-catenin by Axin-bound CK1α and GSK3β [25, 285, 286]. CK1α phosphorylates β-catenin on Ser45, while Ser33, Ser37, and Thr41 are phosphorylated by GSK3β. Those modifications allow the recruitment of β-TrCP that will target ubiquitination and proteasomal degradation of β-catenin [26], leading to constitutive low amount of β-catenin. b Active Wnt pathway 1: When a canonical Wnt ligand binds to Fz receptors at the level of their cystein rich domains (CRD) and to LRP6 coreceptors, the serine/threonine protein phosphatases PP1 and PP2A dephosphorylate Axin and β-catenin, respectively [28]. Concomitantly, Fz recruits Dishevelled (Dsh) at the plasma membrane through its PDZ domain (post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (Dlg1), and zonula occludens-1 protein (Zo-1)). Axin-GSKβ complex is then recruited by LRP5/6 coreceptor via the DIX domain of Dsh, responsible of Dsh polymerization. The plasma membrane-associated GSK3β along with CK1 gamma (CK1γ) phosphorylate LRP5/6 at conserved PPSP motifs (Pro-Pro-Ser-Pro) [29, 287]. This process also requires PFTK and CyclinY [288]. Dsh is also involved in the regulation of GSK3β and Axin internalization by inducing their sequestration in multivesicular bodies [289]. c Active Wnt pathway 2: Following Axin-GSK3β recruitment to the plasma membrane, β-catenin is stabilized by a decrease in GSK3β -mediated phosphorylation through PP2A. Moreover, Dsh can recruit PtdIns 4-kinase type II (PI4KIIa) and PtdIns-4-phosphate 5-kinase type I (PIP5KI) to stimulate the formation of phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) [290]. Both PDZ and DEP (Dishevelled, Egl-10 and Pleckstrin) domains of Dsh interact with PI4KII, whereas its DIX (DIshevelled and aXin) domain binds to and activates PIP5KI. The resulting ternary complex allows PtdIns(4,5)P2 formation, required for clustering and phosphorylation of LRP6. Finally, the trimeric GTP-binding protein Gαo [291] and the βγ-subunits of G protein also participate to Dsh functioning. While βγ-subunits of G protein bind and relocalize Dsh to the plasma membrane, the α-subunit of G protein interacts with and recruits Axin to the plasma membrane, consequently leading to β-catenin stabilization [292, 293]. Gβγ may also participate to the negative feed-back regulation of Dsh activity via the activation of the lysosomal degradation of Dsh in mammalian cells. As a consequence, β-catenin accumulates in the cytoplasm and translocates to the nucleus to bind DNA-bound TCFs and induce transcriptional activation. d Genomic action of β-catenin: TCF are transcription factors that bind to specific response elements, the TCFE (TCF response elements) located at the level of Wnt-responsive promoters. They interact with DNA through their high mobility group (HMG) domain and recruit β-catenin via their catenin-binding domain (CBD). β-catenin bound to TCF recruits Bcl9 and pygopus (pygo) at the level of its N-terminal transactivation domain (NTD) [33, 294, 295]. In addition, Hyrax (Hyx)/Parafibromin binds to the C-terminal transactivation domain (CTD) of β-catenin for activation of target gene expression [34]. Dsh has also been found to participate to transcriptional activation of Wnt target genes via its interaction with β-catenin (and c-jun) [296]. Subsequently, chromatin remodeling enzymes are recruited such as histone acetyltransferases (HATs) CBP/p300 and SET1, and brahma-related gene 1 (BRG1) [35, 297, 298]