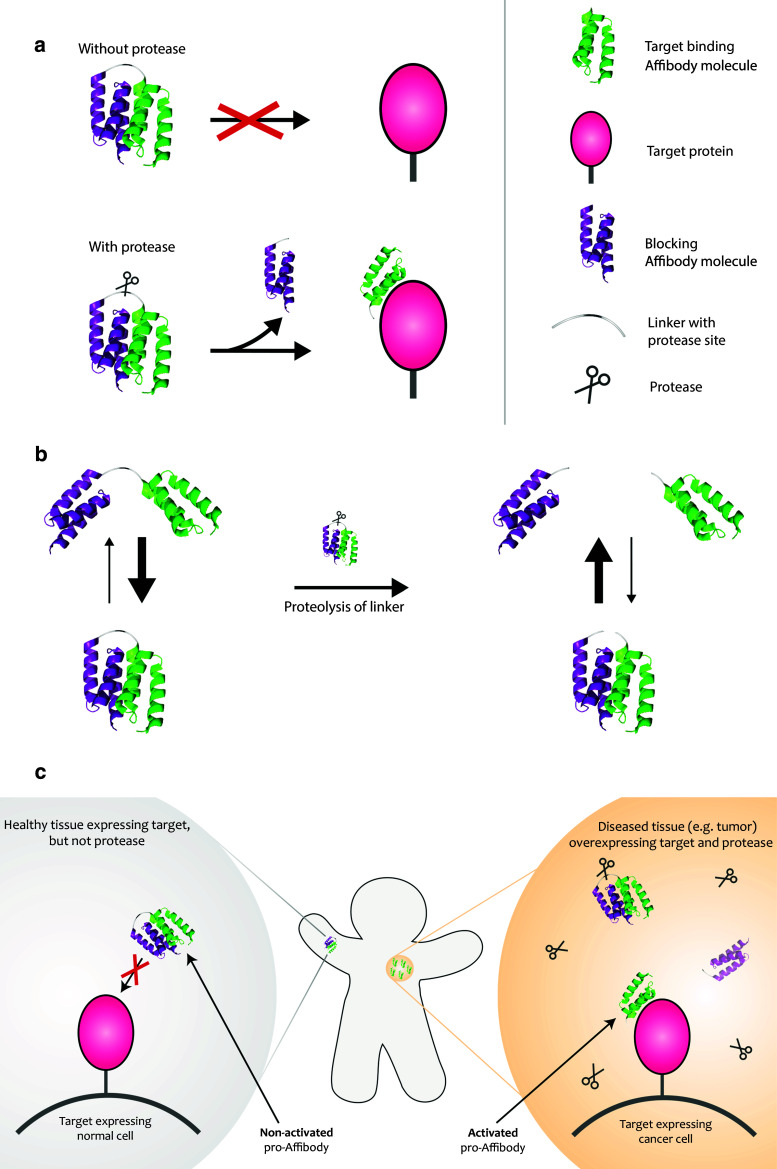
Fig. 1.
Schematic picture describing the pro-Affibody concept. a The pro-Affibody is constructed by fusing a target binding Affibody molecule (green) to an anti-idiotypic Affibody molecule (purple), intended to mask the antigen-binding surface from interacting with its target, via a protease-cleavable linker. The masking prevents target binding until selective proteolysis releases the masking domain and restores binding capacity of the Affibody molecule. b Covalent linkage of the masking domain to the target-binding domain results in a large increase in local concentration, which shifts the equilibrium towards the bound (blocked) state. After proteolysis of the substrate peptide within the linker, the blocking domain can diffuse upon dissociation, which leads to a shift in equilibrium towards the free (active) state. c The target-binding domain within the pro-Affibody will only be able to bind to its target when the pro-Affibody is activated by proteases that are upregulated in diseased tissue, potentially reducing side effects caused by on-target, but off-tissue binding