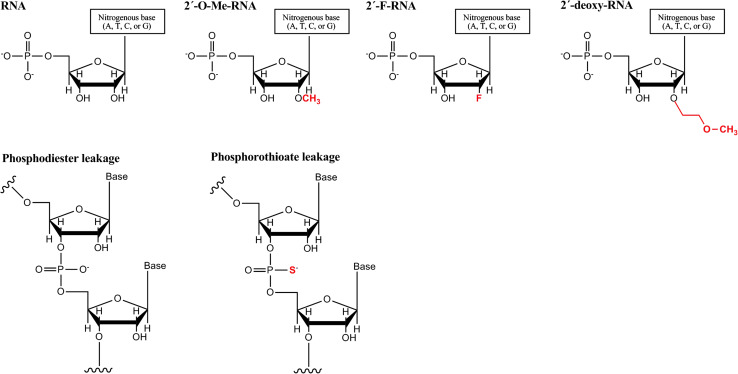
Fig. 2.
The most common chemical modifications applied to siRNAs. a The hydroxyl group on position 2 of ribose has been replaced by 2′-O-methyl, 2′-fluoro or 2′-O-methoxyethyl groups and b the phosphodiester leakage can be modified as a phosphorothioate bond, which retains the negative charge of the phosphate group. Adapted from [30]