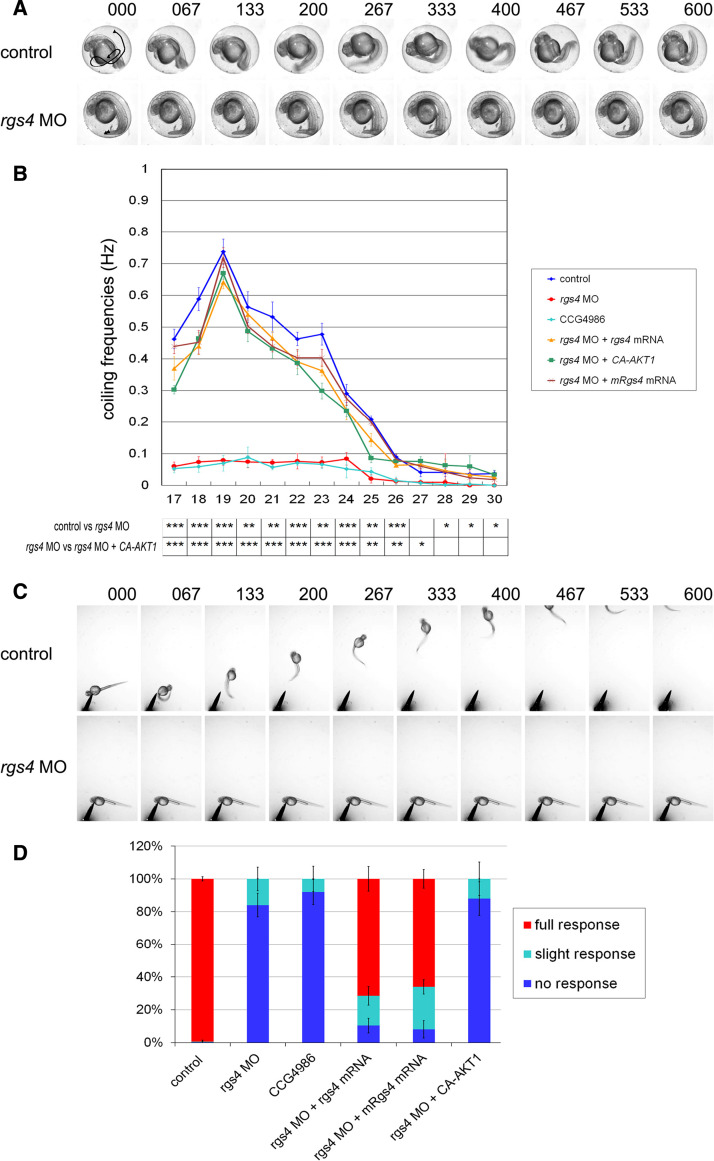
Fig. 3.
Rgs4 knockdown resulted in locomotor phenotypes. a Spontaneous coiling behavior is defective in rgs4 morphants. Individual frames from a time-lapse video showing two consecutive coils by a control embryo at 24 hpf (top panels). Time of each frame is shown at the top. Tail movement is shown by a solid line in the first panel by tracking analyses. Both the frequency and angle of tail coiling is reduced in rgs4 morpholino-injected embryos (lower panels). b Kinetic analysis of the spontaneous coiling between 17 and 30 hpf. The frequency of coiling was reduced at all stages in rgs4 morphants (red) and CCG-4686 treated embryos (cyan) compared with the controls (blue). The defect in coiling frequency in rgs4 morpholino injected embryos could be rescued by concomitant injection of either zebrafish rgs4 (orange), mouse Rgs4 (brown), or ca-AKT1 (green) cRNA. Each data point represents the average frequency of contractions and standard errors for all freely moving embryos examined. Note that all p values were significant before 26 hpf. *p < 0.05, **p < 0.01, ***p < 0.001. c Video capture of aberrant touch and swim responses in Rgs4 knockdown embryos at 48 hpf. Tactile stimuli were applied at a region close to trigeminal ganglia. Control embryos responded to stimuli with a rapid escape response contraction followed by swimming. In contrast, Rgs4 morphants did not respond and were not able to escape. Times indicated on the top are milliseconds. d Proportion of embryos swimming after a tactile stimulus. Aberrant responses were observed in rgs4 morphants and CCG-4686 treated embryos whereas injection of zebrafish rgs4 or mouse Rgs4 cRNA in rgs4 morphants could restore the touch and swim responses. Note that injection of ca-AKT failed to rescue the touch and swim phenotype in Rgs4 deficient embryos. Measurements in (b) and (d) were performed on 20 embryos per batch, and 3 batches were used per time point. rgs4 MO, rgs4 morpholino; rgs4 zebrafish rgs4; mRgs4, mouse Rgs4