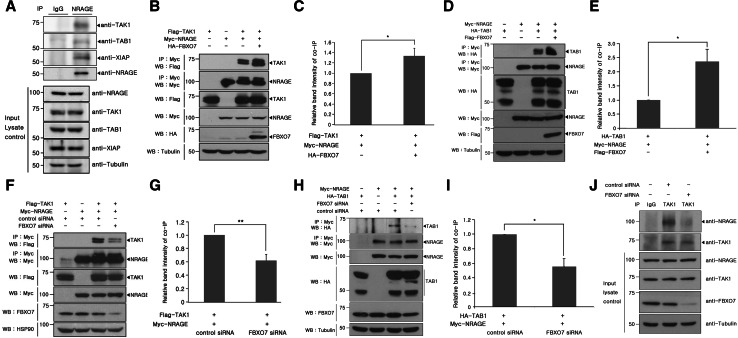
Fig. 5.
FBXO7 promotes formation of the TAK1–TAB1–NRAGE complex. a HEK293 cell lysates were immunoprecipitated with either anti-NRAGE antibody or rabbit preimmune IgG, followed by immunoblotting with the indicated antibodies. Tubulin was used as a loading control. b, d Where specified, HEK293 cells were transfected for 24 h with plasmids encoding Myc-tagged NRAGE, Flag-tagged TAK1 (b), or HA-tagged TAB1 (d) alone or in combination with either HA-FBXO7 (b) or Flag-FBXO7 (d). Cells were immunoprecipitated with the anti-Myc antibody, followed by immunoblotting with anti-Flag or HA antibodies. Proper expression of transfected proteins in cell lysates was examined using immunoblotting, as indicated. To determine equal loading, cell lysates were analyzed by immunoblotting with the anti-tubulin antibody. c, e Relative band intensity levels in the blots of (b) and (d) were quantified by three independent experiments. f, h Where specified, HEK293 cells were transfected for 48 h with plasmids for Myc-tagged NRAGE, Flag-tagged TAK1 (f), or HA-tagged TAB1 (h) alone or in combination with either FBXO7-siRNAs or control siRNAs. Co-IP experiments were performed as described in (b) and (d), respectively. The multiple bands in overexpressed TAB1 lanes may result from differential post-translational modifications and/or irreversible protein cleavage during the sample preparation. g, i Relative band intensity levels of the blots in e and g were quantified by three independent experiments. j HEK293 cells were transiently transfected for 48 h with FBXO7-siRNAs or control siRNAs. Immunoprecipitation of each cell lysates was performed with the anti-TAK1 antibody, followed by immunoblotting with the anti-NRAGE antibody. As a control, cell lysates were immunoprecipitated with rabbit preimmune IgG, as indicated. Endogenously expressed proteins in cell extracts were examined by immunoblotting with the appropriate antibodies. To examine equal loading, cell lysates were analyzed by immunoblotting with the anti-tubulin antibody