Fig. 2.
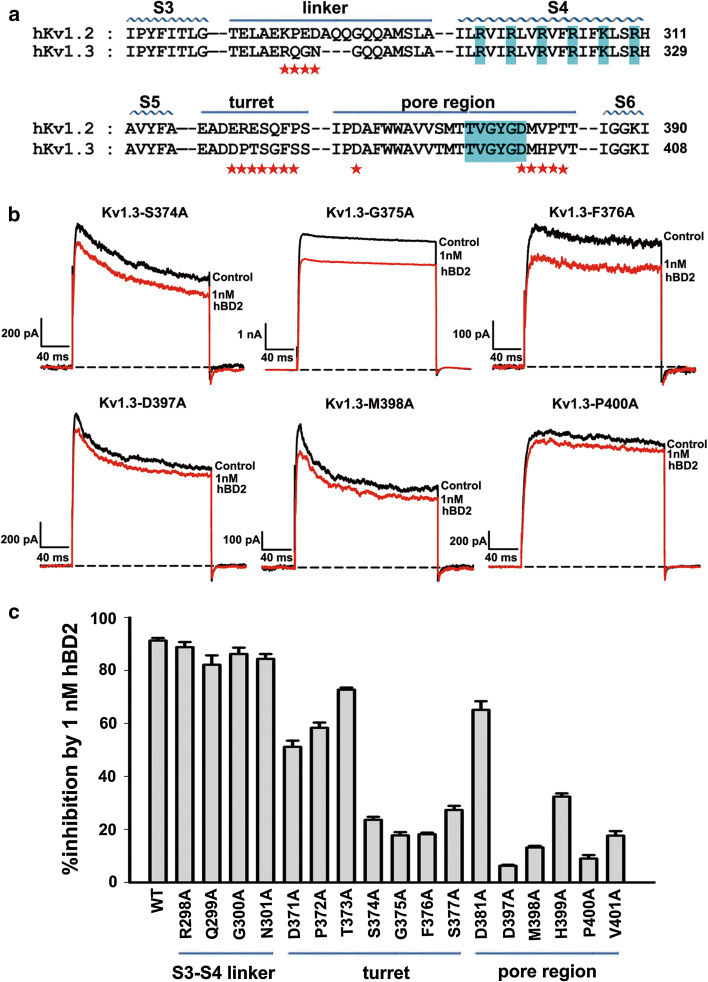
Kv1.3 channel outer vestibule responsible for hBD2 binding. a The amino acid sequence alignment for toxin-interacting regions of human Kv1.2 and Kv1.3 channels. Asterisks indicate amino acid residues of Kv1.3 channel that were changed in later functional studies. b Representative current traces of Kv1.3 channel mutants from the pore region in response to 1 nM hBD2. 1 nM hBD2 inhibited potassium currents of 23.6 ± 1.2 % for Kv1.3-S374A channel, 17.8 ± 1.2 % for Kv1.3-G375A channel, 18.2 ± 0.6 % for Kv1.3-F376A channel, 6.3 ± 0.4 % for Kv1.3-D397A channel, 13.2 ± 0.6 % for Kv1.3-M398A channel and 9.0 ± 1.3 % for Kv1.3-P400A channel, respectively. The black and red lines represent control and measured currents in the absence and presence of hBD2, respectively. c Averaged inhibition of wild-type and mutant Kv1.3 channel currents by 1 nM hBD2. The control currents amplitude in each experiment was fixed as 1 for the normalized currents and inhibition rates were compared. Each channel was tested at least three times. Results are shown as the mean ± SE