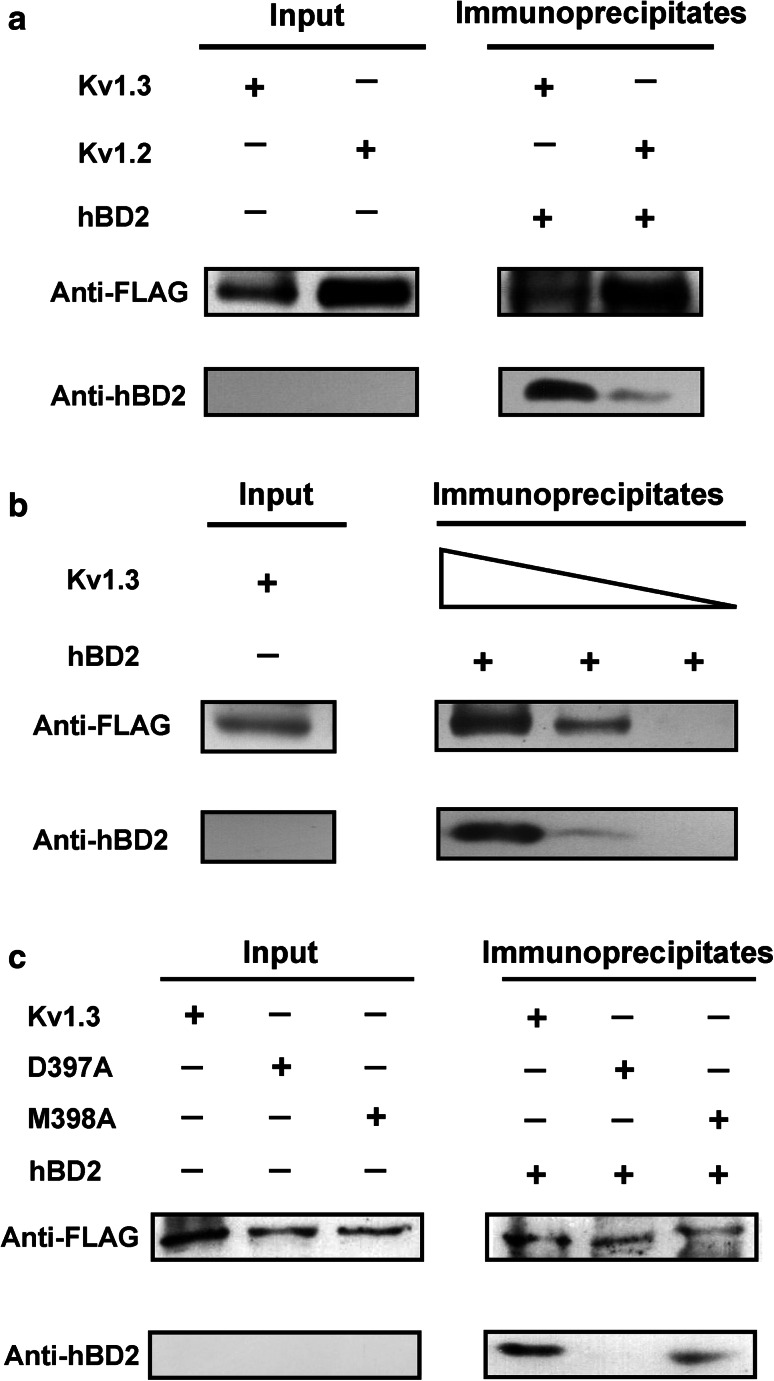
Fig. 3.
The binding of hBD2 to human potassium channels as evidenced by co-immunoprecipitation assay. a Co-immunoprecipitation of Kv1.3 and Kv1.2 channels with hBD2. The complexes formed by hBD2 and potassium channels from HEK293 cell lysates were immunoprecipitated with the anti-FLAG antibody before western blotting analysis using anti-FLAG or anti-hBD2 antibody to detect potassium channel or hBD2 protein, respectively. Complexes were observed with both Kv1.2 and Kv1.3 channels, but Kv1.2 channel retained much less hBD2 than Kv1.3 channel. b The binding of hBD2 to Kv1.3 channel is dose-dependent. The Kv1.3 channel was analyzed for hBD2 binding as described in (a) using protein G-Sepharose beads with decreasing substitution (wedges depict twofold concentration range). c Mutant Kv1.3-D397A and Kv1.3-M398A channels bound less avidly to hBD2. Purified wild-type (Kv1.3) and mutant (Kv1.3-D397A and Kv1.3-M398A) proteins were analyzed for immunoprecipitation with hBD2 as described in (a). Mutant Kv1.3-D397A and Kv1.3-M398A channels abolished and impaired hBD2 binding, respectively