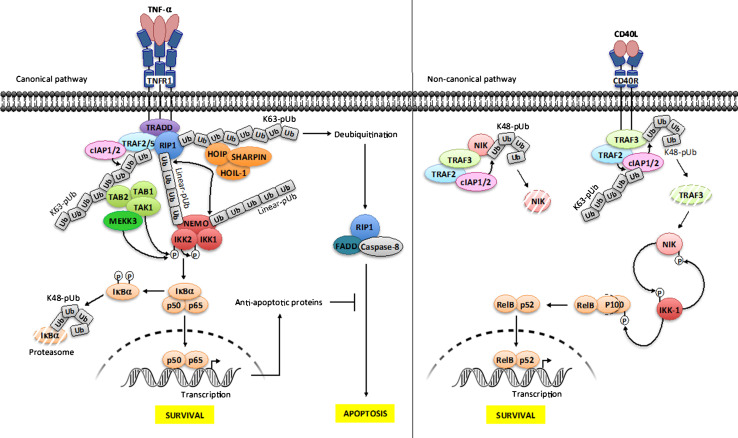
Fig. 2.
Ubiquitination and phosphorylation processes in NF-κB signaling. Canonical NF-κB pathway/TNF-R1 receptor (left panel). The engagement of TNF-α on its cognate receptor induces the recruitment of the adapter TRADD, the E3 ligases cIAP1/2 and TRAF2 and the kinase RIP1. cIAP1/2 catalyze the formation of lys63-linked polyubiquitin (K63-pUb) chains on TRAF2/TRAF5 and RIP1. K63-pUb recruits on one hand the TAB 1–TAB 2–TAK1 kinase complex and on the other hand the LUBAC ligase complex (HOIP, HOIL-1 and SHARPIN), which catalyzes the formation of linear-linked polyubiquitin (linear-pUb) chains on RIP1. Linear-pUb is recognized by NEMO, allowing the recruitment of the IKK complex. TAK1 mediates the activation of IKK-2 by phosphorylation, allowing its auto-phosphorylation, which in turn enables IKK2 to phosphorylate IκB proteins, inducing their degradation by the proteasome and the release of active NF-κB. Another kinase, MEKK3 also participates in this process although its function is less understood. The ubiquitination of NEMO by LUBAC through linear-pUb might amplify NF-κB activation by recruiting more IKK complex. Shortly after signal transmission deubiquitination of RIP1 also results in formation of a FADD-RIP1-Caspase-8 complex, which provokes apoptosis. This process is negatively regulated by a set of anti-apoptotic proteins whose transcription is under NF-κB control. Non-canonical NF-κB pathway/CD40 receptor (right panel). In basal condition, cIAP1/2 catalyzes the formation of lys48-linked polyubiquitin chains (K48-pUb) on NIK, leading to its constitutive degradation. Upon activation, TRAF2-TRAF3-cIAP1/2 complex is recruited to the receptor, allowing TRAF2 to ubiquitinate cIAP1/2 through K63-pUb. This modification on cIAP1/2 induces a switch on its K48 ubiquitin ligase activation from NIK to TRAF3. The consecutive degradation of TRAF3 allows the accumulation of newly synthesized NIK, which in turn phosphorylates IKK-1. Then, IKK-1 phosphorylates and marks p100 for processing through degradation, releasing relB-p52 that translocates to the nucleus for gene activation. As a negative feedback loop, IKK-1 phosphorylates NIK for turning off its activity