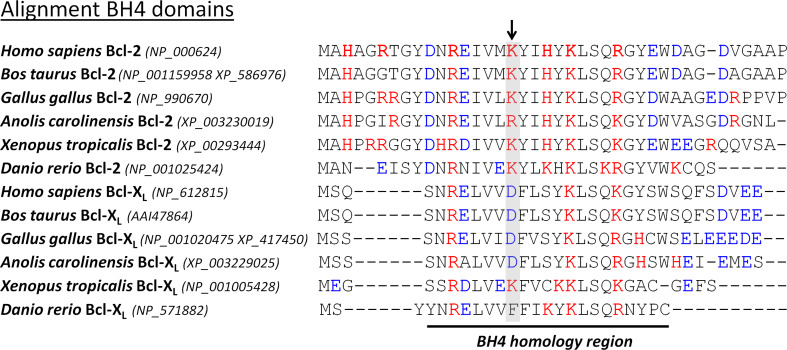
Fig. 3.
Sequence alignment of the BH4 domain of different Bcl-2 and Bcl-XL homologues in the different classes of vertebrates. Bcl-2 and Bcl-XL homologues were obtained from the DeathBase [152]. The number between brackets indicates the accession number of the protein database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/protein). This analysis reveals that the amino acid Lys17 in human Bcl-2 is conserved as a positively charged residue during evolution. The amino acid Asp11 in human Bcl-XL also seems conserved as a negatively charged residue during evolution, although Xenopus Bcl-XL contains a Lys and zebrafish Bcl-XL contains a Phe in the corresponding position. The positively charged (red) and negatively charged (blue) amino acids are depicted in color. The conserved critical Lys residue in the Bcl-2 homologues and its corresponding residue in the Bcl-XL homologues are displayed on a gray background and are indicated by an arrow