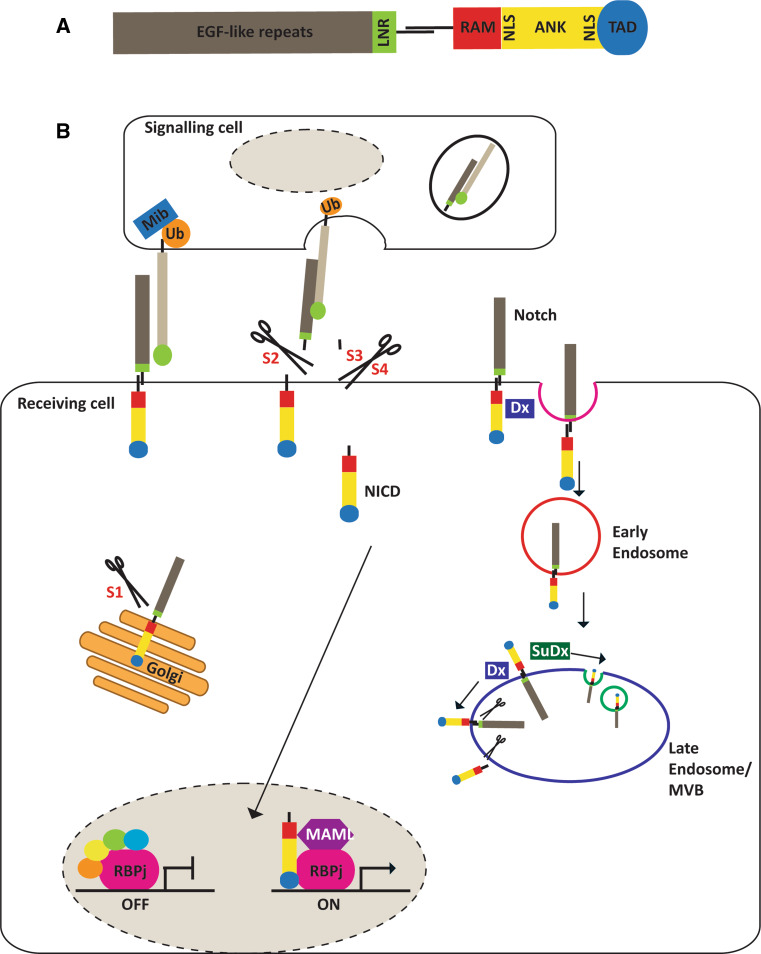
Fig. 1.
Notch signalling pathway. a. Schematic of mammalian Notch proteins. The extracellular domain contains the EGF-like repeats that bind ligand and the LIN12-Notch repeats (LNR) that prevent premature receptor activation. The main regions of the intracellular domain are the RAM23 (RAM) domain that is required for the interaction with members of the RBPj family of transcription factors, the ankyrin repeats (ANK) that bind the Mastermind (MAML) family of co-activators, two nuclear localisation sequences (NLS), and a transcriptional activation domain (TAD). b. The mammalian Notch receptors are synthesised in the ER as a co-linear precursor which is cleaved by a Furin-like convertase at site 1 (S1) within the Golgi. Cleavage results in two non-covalently associated subunits expressed at the cell surface. Notch signalling is triggered by ligand binding to the EGF-like repeats (grey), exposing a second cleavage site (S2) processed by ADAM metalloproteases, generating a Notch intermediate. Two more cleavages occur at site 3 and 4 (S3 and S4) of the transmembrane domain by γ-secretase, releasing the Notch intracellular domain (NICD). NICD migrates to the nucleus and binds to RBPj transcription factors displacing co-repressors and recruiting activators, such as Mastermind. Endocytic trafficking of Notch ligands, promoted by E3 Ubiquitin (Ub) ligases Mindbomb (Mib) and Neuralized (not shown) regulate productive ligand–receptor interactions. Ligand-independent Notch activation can also occur by Deltex (Dx) promoting the endocytosis and trafficking of Notch through the early endosome and its cleavage on the outer membrane of the multivesicular body (MVB). Suppressor of Deltex [Su(Dx)] counters Dx activity and promotes lysosomal degradation of NICD