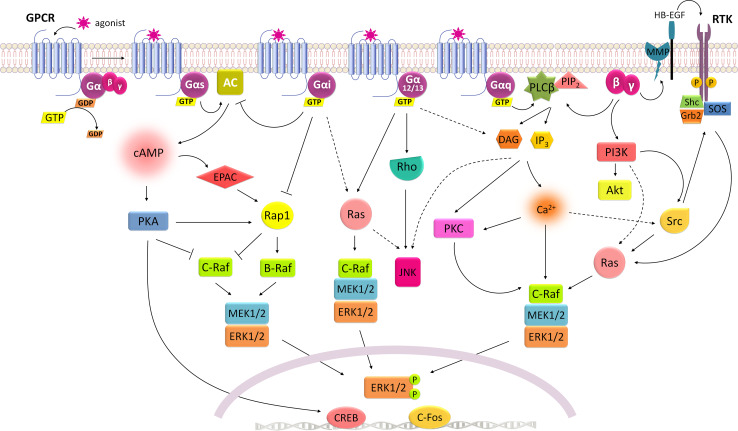
Fig. 1.
Integrated mechanisms for G-protein-mediated signaling. Gs signaling is mainly conveyed through activation of AC/cAMP/PKA pathway, which inhibits C-Raf, but also leads to the activation of Rap1 that relays in B-Raf stimulation of ERK1/2 signaling. Gi inhibits AC activity blocking its inhibitory effect on C-Raf and concomitantly stimulates ERK1/2 signaling by a Ras-dependent mechanism. G12/13 strongly stimulates JNK activity but can also activate ERK1/2 through Ras or by DAG/PKC pathway. Gq can stimulate ERK1/2 via PLCβ/DAG/PKC as well as PLCβ/IP3/Ca2+-signaling mechanisms, either by direct phosphorylation of C-Raf by PKC or by a Ras-dependent manner, which may involve the recruitment of Src. Besides α-subunit, the complex βγ released from α subunit during GPCR activation is also able to promote ERK1/2 signaling through stimulation of PLCβ or PI3K. PI3K usually relays in Akt phosphorylation, but also leads to Src and/or Ras stimulation of ERK1/2. In addition, G-proteins can mediate the transactivation of RTK through its βγ-subunits. Activation of PI3K leads to Src-mediated receptor tyrosine kinase phosphorylation and subsequent recruitment of Shc, Grb2 proteins and SOS to stimulate Ras activity and ERK1/2 phosphorylation. RTK transactivation mediated by βγ-subunits may also occur through an inside-out model. This mechanism is well described for the epidermal growth factor (EGF) receptor, in which βγ activates matrix metalloprotease (MMP) proteins that cleave the ectodomains of membrane-bound growth factors (HB-EGF) to generate soluble EGF ligands that are released from the cell to activate its RTK. Whatever the mechanism of ERK1/2 activation is, these kinases are then able to phosphorylate a wide variety of cytoplasmic and nuclear targets. When translocating to the nucleus, ERK1/2 initiate gene transcription by phosphorylating several transcription factors like CREB and c-Fos