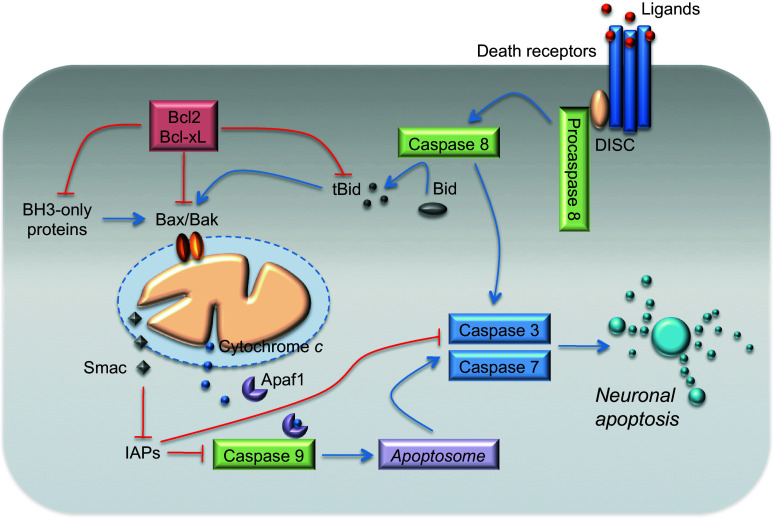
Fig. 1.
The extrinsic and mitochondrial (intrinsic) apoptotic pathways. Apoptosis proceeds through one of two signaling cascades termed the extrinsic and mitochondrial (intrinsic) pathways. The extrinsic pathway is initiated by the ligation of transmembrane death receptors with their ligands, leading to the recruitment of adaptor molecules and to the formation of the pro-caspase-8 DISC. This results in the dimerization and activation of caspase-8, which can either directly cleave and activate caspase-3 and caspase-7, or cleave BH3-interacting domain death agonist (Bid), which in turn engages mitochondrial apoptotic signaling. Truncated Bid promotes the activation of Bax (Bcl-2-associated X protein) and Bak (Bcl-2 antagonist/killer-1), causing outer mitochondrial membrane (OMM) permeabilization. Antiapoptotic proteins, Bcl-2 (B cell lymphoma-2) and Bcl-xL (Bcl-2-like1), prevent OMM permeabilization by sequestering BH3-only proteins, or active Bax and Bak. BH3-only proteins promote Bax and Bak activation. The mitochondrial (intrinsic) pathway is activated by stimuli that trigger the OMM permeabilization and the release of proapoptotic proteins, such as Smac and cytochrome c, from the mitochondrial intermembrane space into the cytosol. Cytochrome c binds to apoptotic protease-activating factor 1 (Apaf-1), inducing its heptamerization and forming the apoptosome that recruits and activates caspase-9. Caspase-9 cleaves and activates the executioner caspase-3 and caspase-7. Smac inhibits the inhibitors of apoptosis proteins (IAPs). See text for a detailed description