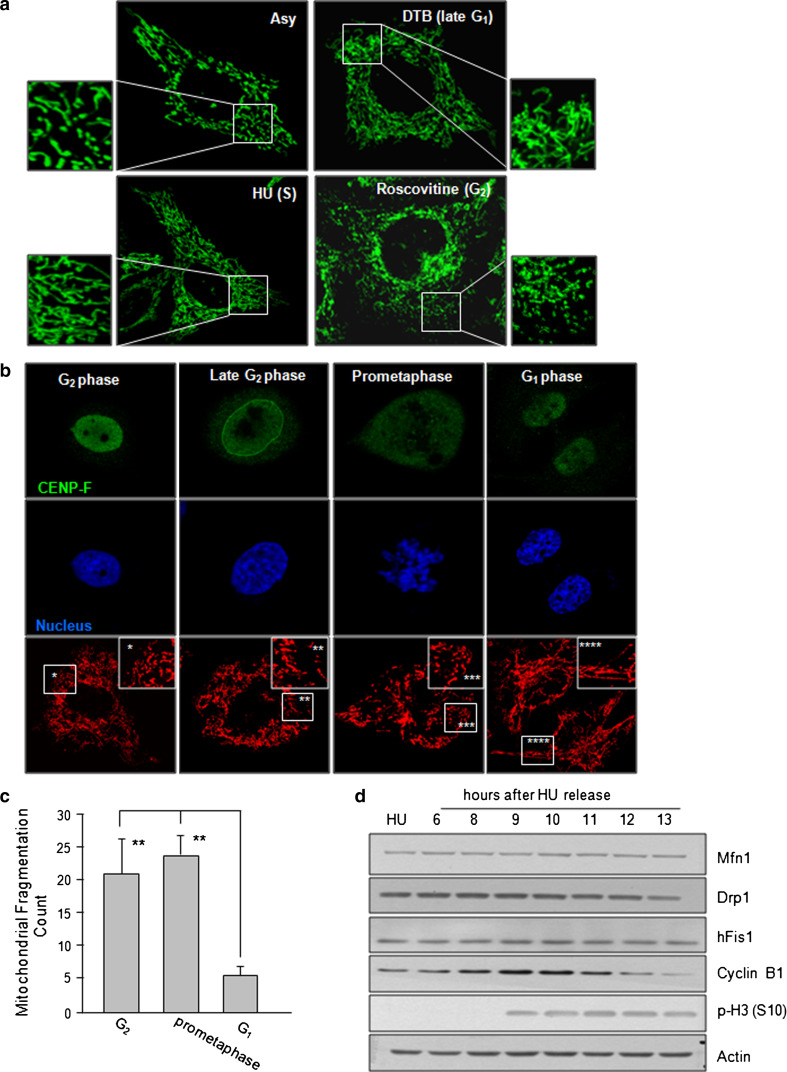
Fig. 1.
Representative mitochondrial morphodynamics during cell cycle. a HeLa cells were synchronized at each phase (G1/S, S, or G2) by the double thymidine block (DTB) method or treatment of 2 mM hydroxyurea (HU) and 100 μM of roscovitine, respectively. Mitochondria were visualized by staining with Mitotracker Green under confocal microscopy. b Cells were synchronized at G1/S boundary by the DTB. At 10 h after the DTB release, cells were fixed in 4 % paraformaldehyde, and mitochondrial morphology and CENP-F localization were visualized by MitoTracker Red staining or immunofluorescence staining against CENP-F. Nucleus was stained with 4′,6-diamidine-2-phenylindole (DAPI) and the images were captured under confocal microscopy. c Acquired mitochondria images of Fig. 1b were applied to image (j), following background subtraction, filtration, threshold, and binarization. The mitochondria fragmentation count was then obtained by counting the particles after normalization to the total mitochondria pixels as shown previously [20]. d Cells were harvested at each time point after HU release. Expressions of mitochondrial fusion and fission regulators were analyzed by immunoblotting. **p < 0.05 by Student’s t test