Fig. 3.
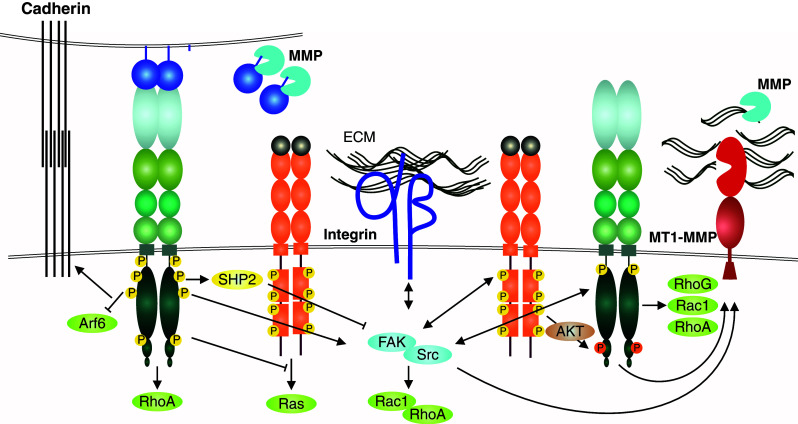
Growth factor and adhesion receptor interactions with EphA2 in tumor cell invasion. The context-dependent, often tumor-suppressive, EphA/ephrinA forward signaling supports the maintenance of cadherin junctions abundant in noninvasive epithelial tumors, and involved also in collective cell invasion (left) [63, 157]. In invasive cancer cells, the ligand-independent EphA2 signaling involves physical interaction with EGFR and Akt-mediated EphA2 phosphorylation at Ser897 (right) [32, 100, 101, 103]. This RTK crosstalk between the Eph and growth factor RTKs is linked to the integrin-mediated cell–ECM communication through intracellular pathways via non-receptor tyrosine kinases Src and FAK [22, 171]. These kinases are common mediators of both upstream and downstream RTK and integrin signals regulating cell adhesive, mitogenic, and migratory responses, including Rho family GTPase-mediated cytoskeletal rearrangements (through e.g., RhoA, Rac1, and RhoG). The integrin-mediated adhesion and MT1-MMP-mediated degradation of the ECM, both required for efficient mesenchymal or collective tumor cell invasion across collagen-rich tissues, ephrin cleavages by MMPs as well as EphA2-dependent MT1-MMP induction are also depicted [13, 22]. RhoA-mediated repulsive responses upon EphA2 activation, limited ECM proteolysis, or impaired integrin-mediated adhesion can instead lead to more amoeboid-type single cell invasion by increased actomyosin contractility (see also Fig. 4b)
