Fig. 4.
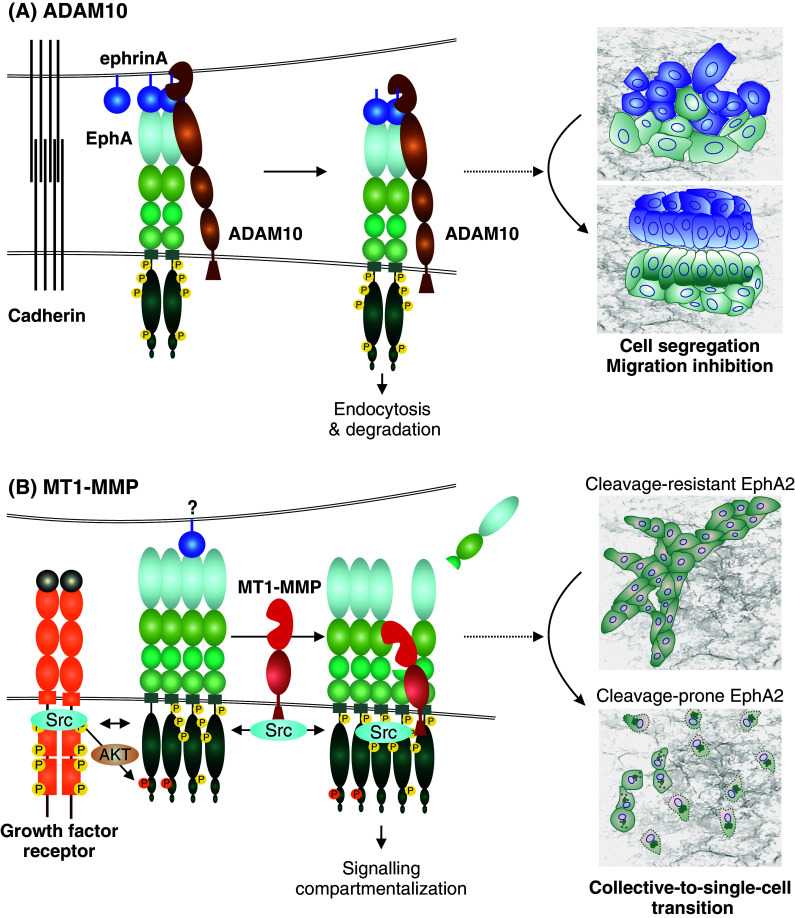
Metalloproteinase regulation of Eph signaling and cellular responses. a EphrinA-EphA binding upon cell–cell contact induces activation and conformational changes in EphA, leading to the recruitment of ADAM10. The Eph-interacting ADAM10 then cleaves receptor-bound ephrinA ligands in trans from an adjacent cell, followed by endocytosis of the activated receptor-ligand complexes, and cell–cell repulsion or contact inhibition of locomotion [21]. As typical for RTKs, the transiently activated signal can be attenuated by receptor degradation after endocytosis. This Eph forward signaling leads to cell segregation and migration inhibition. b In invasive carcinoma cells, EphA2 overexpression and ephrinA suppression are frequently coupled with the induction of MT1-MMP [22]. In these cells, growth factor receptors such as ErbB2/EGFR are also often induced or mutationally activated to mediate ligand-independent EphA2 crosstalk as well as migration signaling through Src. In this context, Src activities can also promote the transcriptional activation and phosphorylation of MT1-MMP [198]. EphA2 kinase activity-independent cleavage of the adjacent EphA2 receptor in cis by MT1-MMP triggers Src and EphA2 activity-dependent intracellular translocation of the EphA2 signaling complexes and subsequent RhoA GTPase activation [22]. When coupled with the Src-mediated migration signaling, these events and the intracellular signaling compartmentalization lead to cytoskeletal contractility, cell–cell repulsion, and a switch from collective to single-cell invasion [22]
