Fig. 5.
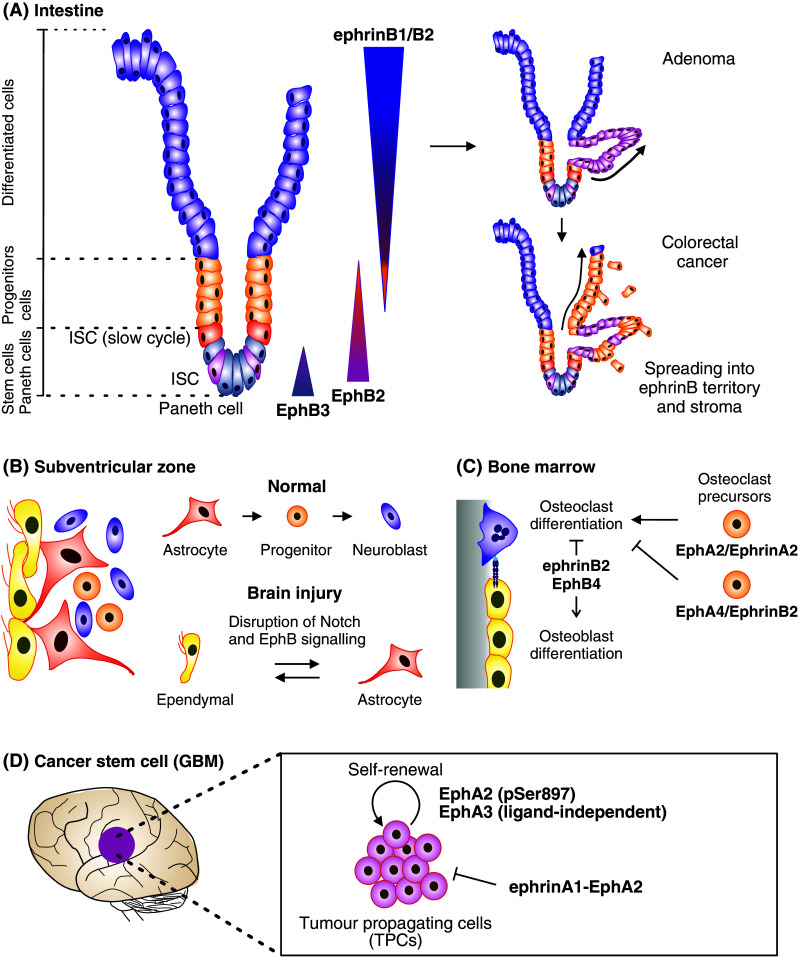
Eph signaling in stem cell dynamics. a EphrinB–EphB signaling regulates both proliferation and migration in intestinal stem cell niche [159, 254]. Intestine stem cells (ISC) reside together with Paneth cells at the bottom of the crypt, where they divide and give rise to differentiated progenitor cells. As these cells differentiate, they migrate up the crypt and villus to sustain renewal of the intestinal epithelium. At the bottom, Paneth cells express EphA3, whereas ISC and progenitor cells express EphB2 in a gradient that decreases towards the lumen. EphrinB1 and ephrinB2 are expressed by the differentiated epithelial cells in a counter gradient [159]. EphB2/ephrinB1 bidirectional signaling mediate repulsive responses required for cell compartmentalization along the crypt-villus axis [159, 253]. In early stage colorectal cancer (adenoma), Wnt pathway enhances the expression of EphB2/EphB3, leading to epithelial evaginations and development of adenomatous polyps (right top) [159]. Along progression to colorectal cancer, the EphB expression is lost allowing spread of tumor cells into ephrinB-expressing areas as well as into surrounding stroma (right bottom) [223]. b EphrinB–EphB signaling regulates lineage plasticity of adult neural stem cell niche cells. The subventricular zone (SVZ) of the brain lateral ventricles contains a single layer of multiciliated ependymal cells lined by differentiated niche astrocytes and ventricle contacting self-renewing astrocytes. In physiological conditions, ependymal cells are involved in maintaining the SVZ stem cell niche, while self-renewing astrocytes are able to differentiate into neural stem cells (progenitors) and further to neuroblasts. Ependymal cells express EphB1/EphB2 receptors and ephrinB1/ephrinB2 ligands, while SVZ astrocytes express EphB1 and ephrinB2 [249]. EphB forward signaling downstream of Notch contributes to the maintenance of ependymal cell and astrocyte characteristics [250, 257]. Upon brain injury, the Notch–EphB signaling is disrupted by EphB downregulation, resulting in cell lineage interconversion between ependymal cells and astrocytes [249]. c In bone marrow, Ephrin–Eph signaling regulates osteoclast differentiation and osteoclast-osteoblast communication [267, 268]. Bone remodeling is sustained by a balance between new bone formation by osteoblasts and old mineralized bone resorption by osteoclasts. Within osteoclast precursors EphA4-dependent ephrinB2 reverse signaling limits, whereas ephrinA2–EphA2 signaling promotes osteoclast differentiation. Upon interaction with EphB4 expressing osteoblasts, EphB4 forward signaling mediates osteoblast differentiation and bone formation, whereas ephrinB2 reverse signaling inhibits bone resorption. d EphA2 and EphA3 signaling maintains cancer stem cell characteristics in human glioblastoma multiforme (GBM) [10, 11]. These tumors are composed of heterogeneous populations of differentiated dividing tumor cells and less differentiated tumor-propagating cells (TPCs). Ligand-independent EphA2 signaling coupled with Ser897 phosphorylation, and ligand-independent EphA3 signaling maintains TPCs in an undifferentiated state, further promoting their self-renewal and tumor-propagating abilities
