Fig. 4.
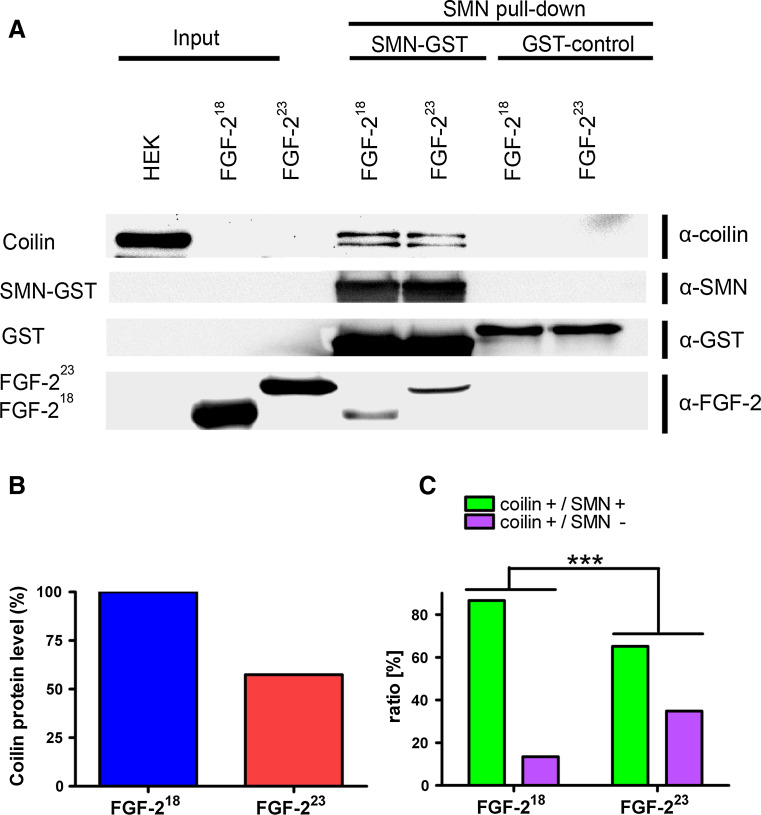
FGF-223 reduces SMN binding to coilin. a Endogenous coilin was purified from lysates of HEK293T cells using recombinantly expressed FGF-223, FGF-218 and GST fusion of SMN coupled to glutathione beads (SMN-GST) and analyzed by Western blot. GST was used as a control (GST control). FGF-223 decreased binding of coilin to SMN-GST. Because of the high amounts of FGF-218 used, a slight unspecific binding of FGF-218 to SMN-GST was found. After SMN-GST pulldown, also fragments of coilin (anti-coilin) and fragments of SMN-GST (anti-GST) were detected. b The level of endogenous, SMN-bound coilin was decreased in lysates of cells with FGF-223 (densitometrical analysis of the highest band of coilin). c To determine changes of coilin-SMN association in CBs in vivo, HEK293T cells were transfected with pFGF-223-DsRed2 (d–d’) or pFGF-218-DsRed2 (as a control, e–e”). Cells were fixed and immunostained with anti-coilin (d3–e3”) and anti-SMN (d2–e2”). Nuclei were analyzed by counting SMN-positive or -negative CBs (FGF-218: n = 119, FGF-223: n = 132) as demonstrated in the merged pictures (d4–e4”). The distribution of SMN-positive (yellow arrows) and -negative (red arrows) CBs was significantly changed after overexpression of FGF-223-DsRed2 (c, ***p < 0.001, χ 2 test)