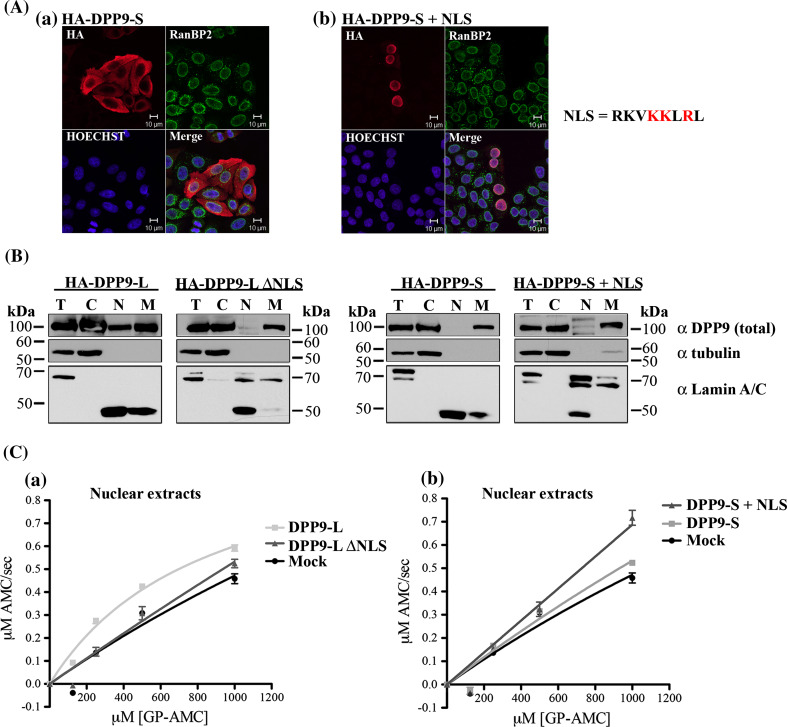
Fig. 5.
The NLS sequence RKVKKLRL is sufficient to target DPP9 to the nucleus, where it is active. a HA-tagged DPP9-S (HA-DPP9-S) and DPP9-S fused to the NLS of long (RKVKKLRL) at its N-terminus (HA-DPP9-S + NLS) were overexpressed in HeLa cells and subjected to indirect immunofluorescence, detecting the HA tag and simultaneously RanBP2, a marker protein for the nuclear rim. Nuclei were visualized by Hoechst. Shown is a representative image from at least three independent experiments. b HeLa cells were transfected with the corresponding constructs and subjected to subcellular fractionation (T totals, C cytosolic fraction, N nuclear fraction, and M membranous fraction). A total of 10 μg of each fraction was analyzed by Western blotting using the commercial Abcam DPP9 (total) antibody. The nature of the slower migrating bands (ca. 120 kDa) interacting with the DPP9 antibody may represent putative modification of DPP9. Lamin A/C was used as a marker for the nuclear fraction, tubulin as a marker for the cytosolic fraction. The faster migrating band of Lamin A/C represents a degradation product of this protein. Note that protease inhibitors were omitted from the fractionation procedure to enable subsequent DPP9 activity measurements in the various fractions. Shown is a representative of at least three independent experiments (full scans in supplementary Fig. S3). c A total of 10 μg of HeLa nuclear fractions from (B) was analyzed for GP-AMC hydrolysis. The experiment was performed at least three times, each time in triplicates. Shown is a representative Michaelis–Menten analysis, including error bars