Fig. 6.
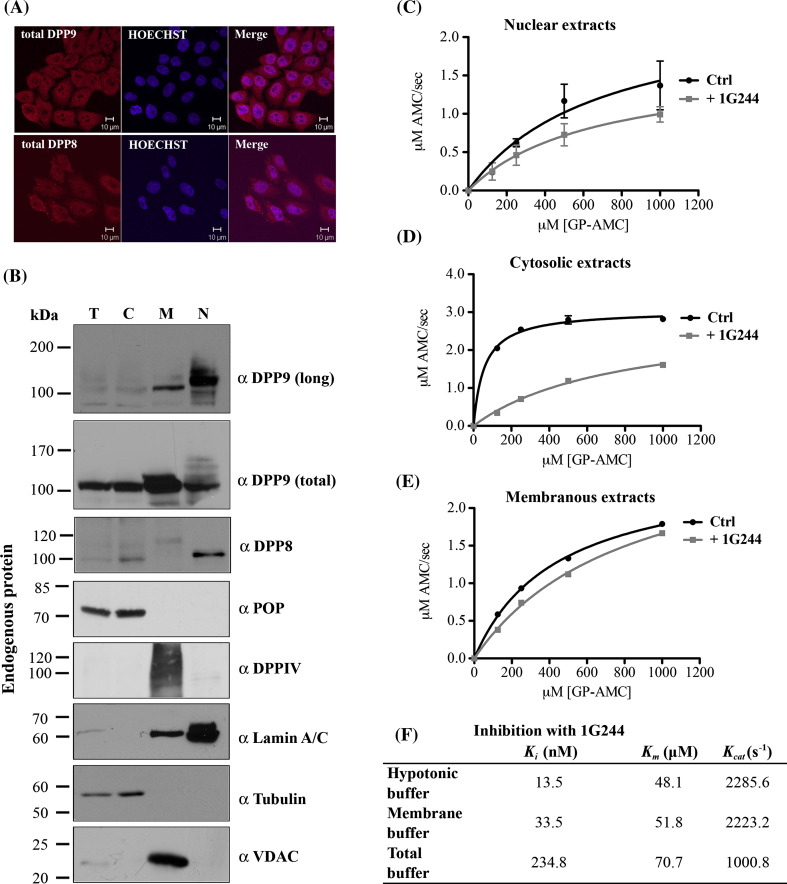
Endogenous DPP9-long localizes to the nucleus and is active there. a Localization of endogenous DPP9 and DPP8 in HeLa cells was analyzed by indirect immunofluorescence using the Abcam commercial antibodies targeting the catalytic domains of these peptidases: α DPP9 (total) (#ab42080) and α DPP8 (#ab42077). Nuclei were visualized by Hoechst staining. Shown is a representative of at least three independent experiments. b HeLa cells were subjected to subcellular fractionation and 10 μg of each fraction were analyzed by Western blotting with the indicated antibodies for members of the DPPIV family: α DPP9 (long), Abcam α DPP9 (total) (#ab42080), α DPP8 (#ab42077) and α DPPIV. Fractions were labeled as follows: T totals, C cytosolic fraction, M membranous fraction, and N nuclear fraction. Fractions were also analyzed for the related enzyme prolyl oligopeptidase: α POP. The following antibodies were used as markers: Lamin A/C for the nuclear fraction, tubulin for the cytosolic fraction and VDAC (voltage-dependent anion channel) for the membranous fraction. Shown are representative blots of at least three independent experiments. c–e DPP activity in the subcellular fractions. All Michaelis–Menten assays were performed at least three times in triplicates. Shown are graphs including error bars. c Hydrolysis of GP-AMC by 5 μg nuclear extracts in the presence or absence of the DPP8/9-specific inhibitor 1G244. d 5 μg of cytosolic extracts were analyzed as in c. e 5 μg of membranous extracts were tested as in c. f Table summarizing the activity of recombinant DPP9-l incubated in the different fractionation buffers: hypotonic buffer (cytosolic fraction), membrane buffer (membranous fraction) and total buffer (nuclear fraction). The table summarizes the effect of the buffers on DPP9 activity (K m and K cat) and inhibition (K i) by 1G244. All assays were performed in triplicates and were repeated at least three times