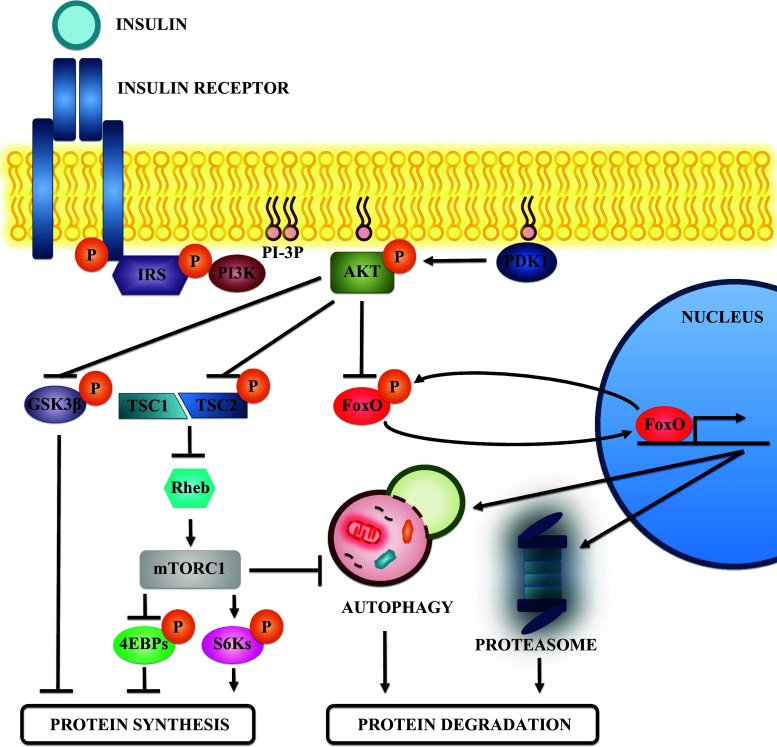
Fig. 1.
Insulin signaling and protein metabolism in skeletal muscle. Schematic view of the effectors that participate in the insulin signaling pathway. Insulin binding to its receptor activates its tyrosine kinase activity and promotes IRS recruitment. Then IRS is phosphorylated and this event generates binding sites for the class I PI3K. PI3K activity generates phosphoinositides that act as binding sites for the recruitment of AKT to the membrane. There, AKT is activated by phosphorylation of other kinases, such as PDK1, and participates in various signaling pathways to promote protein synthesis and inhibit protein degradation. AKT phosphorylates and inhibits GSK3β, a negative regulator of protein synthesis. In addition, AKT phosphorylation also inhibits TSC2, a protein found in a complex with TSC1. The TSC1-TSC2 complex inhibits Rheb by promoting its GTPase activity. Inhibition of TSC2 preserves Rheb function and activates mTORC1. mTORC1 promotes protein synthesis by phosphorylating and inhibiting the 4EBPs, as well as by activating the S6 kinases through phosphorylation. Moreover, mTORC1 inhibits protein degradation by inhibiting autophagy. Finally, AKT also phosphorylates and inhibits the FoxO transcription factors. The FoxOs promote protein degradation by increasing the expression of genes involved in autophagy and in the UPS