Abstract
Regenerative medicine for skeletal and cardiac muscles still constitutes a fascinating and ambitious frontier. In this perspective, understanding the possibilities of intrinsic cell plasticity, present in post-natal muscles, is vital to define and improve novel therapeutic strategies for acute and chronic diseases. In addition, many somatic stem cells are now crossing the boundaries of basic/translational research to enter the first clinical trials. However, it is still an open question whether a lineage switch between skeletal and cardiac adult myogenesis is possible. Therefore, this review focuses on resident somatic stem cells of post-natal skeletal and cardiac muscles and their plastic potential toward the two lineages. Furthermore, examples of myogenic lineage switch in adult stem cells are also reported and discussed.
Keywords: Skeletal muscle, Cardiac muscle, Resident stem cells, Myogenic regeneration, Muscular dystrophy, Lineage switch
From embryo to adult: skeletal versus cardiac myogenesis
During embryonic development, skeletal muscle precursors arise from the paraxial mesoderm. During the process of somitogenesis, somites develop in a cranial-to-caudal sequence from the segmental plate of the paraxial mesoderm and flank the neural tube [1, 2]. Subsequently, proliferative progenitor cells are recruited by delamination from the central dermomyotome, which will form the basis for the growth of the myotome and derived populations such as the satellite cells (SCs) during fetal and postnatal development [3].
Differently from the paraxial mesoderm, the splanchnic mesoderm (visceral or cardiogenic mesoderm) gives rise to the heart. The initial heart tube is orientated along the craniocaudal axis. Then the cardiac looping starts, the heart tube grows rapidly, and primary and secondary heart fields are specified. Progenitors from the primary heart field give rise to both ventricles and atria, and to the atrium-ventricular canal, while secondary heart field progenitors contribute to the formation of the outflow tract and the other cardiac regions, except the left ventricle. After looping, the heart is finally articulated in the four contractile chambers and the myocardium is composed of bi- or tri-nucleated myocytes, electrophysiologically coupled to each other [4].
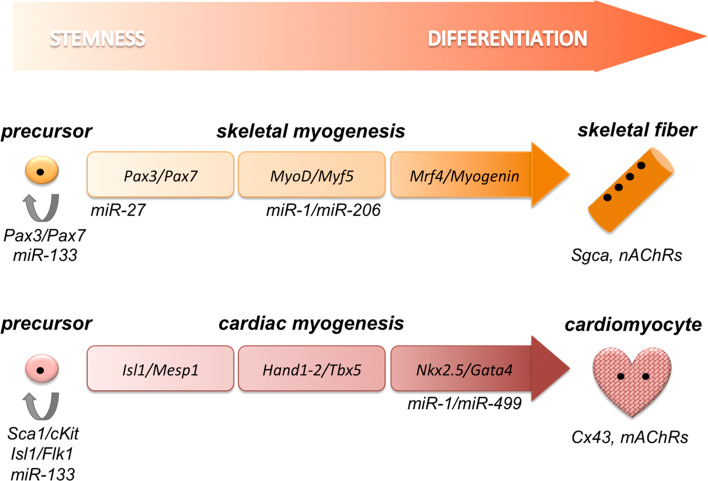
During development, progression through skeletal or cardiac lineage relies on different gene networks, often recapitulated during post-natal regeneration (Fig. 1).
Fig. 1.
Schematic comparison of factors regulating skeletal and cardiac differentiation, as mentioned in the text. Skeletal fibers and cardiomyocytes are distinguished by αSarcoglycan (Sgca) and nicotinic acetylcholine receptors (nAChRs) in the former, and by Connexin43 (Cx43) and muscarinic acetylcholine receptors (mAChRs) in the latter
Skeletal muscle progenitors in the myotome express the paired-domain and homeodomain-containing transcription factors Pax3 and Pax7 [5–7]. These activate the muscle regulatory factors (MRFs), namely Myf5, MyoD, Mrf4, and Myogenin, which subsequently drive the skeletal myogenesis [8, 9].
In contrast to the skeletal muscle, cardiac commitment relies on less hierarchical gene networks. The earliest cardiac specification marker can be traced to mesoderm posterior protein 1 and 2 (Mesp1, Mesp2), markers of primitive heart tube formation and required for cardiac progenitor migration. Mesp1 is able to promote transcription of several other cardiomyogenic markers and, accordingly, in Mesp1-null mice cardiac formation is abolished since the early stage [10]. Recently, Mesp1 has been proposed as a context-dependent determination factor to specify different lineage outcomes, including cardiac cells [11]. In addition, MADS-box factor myocyte enhancer factor-2 (Mef2), in conjunction with other transcription factors, directly activates the expression of genes encoding myofibrillar proteins [12]. Similarly, serum response factor (Srf), a related MADS-box factor, associates with an array of transcription factors including NK2 transcription factor related locus 5 (Nkx2.5), GATA binding protein 4 (Gata4) and Myocardin to control the expression of the contractile apparatus genes, e.g., actin, myosin, and troponins [13]. Furthermore, Mef2C physically interacts with T-box 5 (Tbx5) to regulate early stages of heart development and expression of cardiac myosin, as reported in zebrafish [14]. Intriguingly, over-expression of Gata4, Tbx5 and Mef2C converts murine fibroblasts into functional cardiomyocytes [15]. Finally, Hand1 and Hand2 can be considered to exert the analogous function of Pax3/Pax7, since they control the entry into the cardiac differentiation program. Loss of Hand1 results in increased cardiomyocyte differentiation, whereas Hand1 gain-of-function supports cardiomyocyte proliferation [16].
Stem cell plasticity in the post-natal skeletal muscle
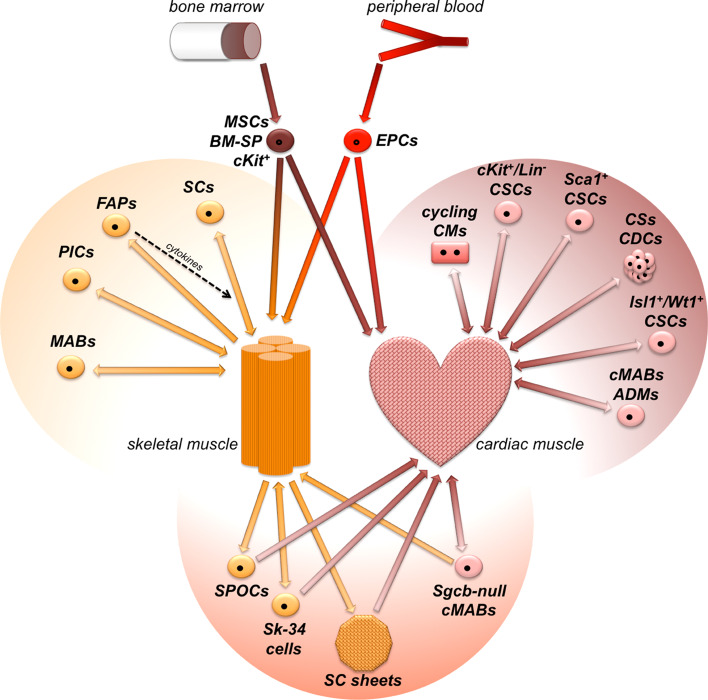
The intrinsic regenerative potential of the post-natal skeletal muscle has been linked to resident precursors since the first observation of quiescent SCs residing under the basal lamina of adult fibers [17]. Later, many somatic stem cells have been isolated with diverse molecular signatures and plasticity grades [18]. Understanding the in vitro/in vivo properties of resident myogenic cell pools, whose subset is reviewed here (Table 1; Fig. 2), is fundamental for advancing our knowledge of post-natal plasticity of the skeletal muscle and for promoting novel therapeutic strategies for cell-mediated myogenic regeneration.
Table 1.
Main characteristics of resident post-natal stem cells in skeletal muscle, with emphasis on species, antigen profile, lineage marker, and in vivo plasticity
| Cell type | Species | Surface antigens | Lineage markers | In vivo plasticity | References | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| SCs | Mouse | M-cadherin, c-Met, CD34 | NA | Pax7 | Skeletal fibers | [19] |
| SCs | Mouse | Desmin | NA | Myf5, MyoD, Pax7 | Skeletal fibers | [21] |
| SCs | Mouse | NA | NA | MRFs | Skeletal fibers | [22] |
| MABs | Dog | CD44, CD13 | CD34, CD45, CD117, CD31 | NA | Skeletal fibers | [41] |
| MABs | Mouse, human | AP, NG2, CD13, CD44, CD49b, CD63, CD90, CD105, CD140b, CD146 | CD31, CD34, CD45, CD56, CD62L, CD71, CD106, CD117, CD133 | NA | Skeletal fibers | [45] |
| MABs | Mouse, human | AP, NG2, Sca1, CD13, CD44, CD49f, CD90, CD140a, CD140b | CD31, CD45, CD56, CD133 | NA | NA | [48] |
| PICs | Mouse | Sca1, CD34 | Pax7 | Pw1 | Skeletal fibers | [50] |
| FAPs | Mouse | CD34, Sca1 | Lin, CD31, CD45, α7integrin | NA | Supportive role for SCs | [51] |
| FAPs | Mouse | Sca1, CD140a | α7integrin | NA | Supportive role for SCs | [52] |
Each entry relates to one reference and the entry order is referred to the review
NA information not available in the cited reference, SCs satellite ells, MABs mesoangioblasts, PICs Pw1+ interstitial cells, FAPs fibroadipogenic progenitors
Table 2.
Species, antigen profile, lineage marker, and in vivo plasticity of post-natal stem cells located in the heart of different origins
| Cell type | Species | Surface antigens | Lineage markers | In vivo plasticity | References | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Lin− CSCs | Mouse | cKit, MDR, Sca1 | Lin, CD45 | Ets1, Gata4, Mef2C | CMs | [60] |
| cKit+ CSCs | Rat | cKit | Lin, CD31, CD8, CD20, CD34, CD45, CD45RO, TER-119 | Nkx2.5, Gata4, Gata5, Mef2 | CMs, SMCs, ECs | [61] |
| cKit+ CSCs | Mouse, human | cKit | CD31, CD90, CD146 | NA | CMs, ECs, fibroblasts | [62] |
| cKit+ CSCs | Human | cKIT | LIN | NKX2.5, GATA4, MEF2C | NA | [63] |
| Lin− CSCs | Rat | cKit, MDR, Sca1 | Lin | Nkx2.5, Gata4, Mef2C | CMs, SMCs, ECs | [64] |
| Lin− CSCs | Human | cKIT, MDR | LIN | MEF2C | CMs, SMCs, ECs | [67] |
| Sca1+ CSCs | Mouse | Sca1, CD31, CD38 | Lin, cKit, Flk1, CD34, CD45 | Gata4, Mef2C, Tef1 | CMs | [69] |
| Sca1+ CSCs | Mouse | Sca1, CD29, CD44, CD34 | cKit, CD31, CD45 | Nkx2.5, Gata4, Mef2C | CMs | [70] |
| Sca1+ CSCs | Mouse | Sca1 | cKit, CD31, CD45 | Nkx2.5, Gata4, Mef2C | NA | [71] |
| Sca1+ CSCs | Mouse | Sca1 | CD31, CD45 | Nkx2.5, Gata4 | CMs, ECs | [72] |
| CSs | Mouse, human | Sca1, cKit, Flk1, CD31, CD34, | NA | NA | CMs, SMCs, ECs | [73] |
| CDCs | Pig, human | cKIT, CD31, CD34, CD90, CD105 | LIN, MDR, CD45, CD133, | NKX2.5 | CMs, ECs | [74] |
| CDCs | Rat, human | cKit, Ddr2, MHC-II, CD31, CD90, CD105, CD140b | MHC-I, CD45, CD80, CD86 | NA | CMs, ECs | [75] |
| CDCs | Human | CD105 | CD45 | NA | NA | [76] |
| Isl1+ CSCs | Mouse | NA | cKit, Sca1, CD31 | Isl1, Nkx2.5, Gata4 | NA | [79] |
| Wt1+ CSCs | Mouse | Sca1 | cKit | Wt1, Isl1, Nkx2.5, Tbx18 | CMs | [82] |
| cMABs | Human | AP, NG2, cKIT, CD31, CD34, CD44, CD146 | CD45, CD133 | NKX2.5, GATA4, MEF2A, TBX2, TBX5 | CMs | [83] |
| cMABs | Mouse | AP, NG2, Sca1, cKit, CD31, CD34, CD44, CD140 | CD45 | Isl1, Nkx2.5, Gata4, Gata6, Mef2A, Mef2C | CMs | [84] |
| cMABs | Dog | AP, NG2, cKit, Flk1, CD140a, CD140b | CD31, CD73, CD105 | Gata4, Gata6, Mef2A, Mef2C, Mesp1, Tbx2, Hand2 | CMs | [85] |
| ADMs | Mouse | Sca1, CD34, CD90.1 | CD13, CD31, CD45, CD146 | Nkx2.5 | CMs | [86] |
Each entry relates to one reference and the entry order is referred to the review
NA information not available in the cited reference, CSCs cardiac stem cells, CSs cardiospheres, CDCs cardiosphere-derived cells, cMABs cardiac mesoangioblasts, ADMs aorta-derived mesoangioblasts, CMs cardiomyocytes, SMCs smooth muscle cells, ECs, endothelial cells
Satellite cells are resident stem cells, expressing the surface antigens Syndecan-4, M-Cadherin, CD34, Cxcr4, β1-Integrin and the myogenic factors Pax3 and Pax7 [19]. After injury, SCs enter the cell cycle, rapidly upregulate the expression of MRFs that induce the terminal differentiation to novel myofibers. A small percentage of activated SCs does not undergo myogenic differentiation but instead self-renews, restoring the quiescent pool [20].
Albeit strongly primed to the skeletal myogenesis, it has been reported that SCs can transdifferentiate at little extent toward the adipogenic and the osteogenic lineages in vitro, although contaminations with non-myogenic cells can easily occur [21–23]. In this view, it has been recently reported that SCs can be stimulated to store lipids, but fail in undergoing terminal adipogenic differentiation in vitro [24]. Furthermore, exposure to bone morphogenetic protein (BMP) ligands is not per se sufficient to obtain robust commitment to the osteogenic lineage [25].
SC intrinsic commitment relies on a balanced expression of MRFs. Murine strains carrying ablation of Myf5, MyoD or Mrf4 show only mild myogenic abnormalities, while triple knockout mice completely lack any skeletal muscle [26–29]. Analogously, adult triple knockout murine SCs are unable to differentiate in vitro and in vivo [30]. MyoD-null SCs express low levels of Myogenin and show a dramatic differentiation deficit, demonstrating the importance of the sequential MRF hierarchy to achieve terminal specification [31].
Remarkably, SC self-renewal and commitment are also strongly influenced by post-translational regulations and epigenetic cues. Sirt1 is a NAD+-dependent protein deacetylase [32], which induces premature differentiation of SCs when downregulated [33]. Similarly, primary SCs derived from Sirt1 +/null differentiate precociously and are resistant to anti-differentiation stimuli, such as glucose restriction [34]. Conversely, SC proliferation increases when Sirt1 overexpression inhibits MyoD activity [35]. Furthermore, tumor necrosis factor alpha (TNFα) signaling has been linked to the chromatin remodeling Polycomb Repressive Complex 2 (PRC2) and to p38α kinase, during SC differentiation [36]. During post-injury inflammation, TNFα activates p38α, which favors PRC2 relocation from muscle structural genes to Pax7 regulatory regions, repressing its expression and stimulating differentiation. Accordingly, genetic or pharmacological interference with TNFα, p38α, or PRC2 results in sustained Pax7 expression and SC self-renewal, and this effect is reversible [36].
In addition, regulatory RNAs, such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) modulate the balance between quiescence and commitment in SCs. Skeletal muscle-specific miR-206 is expressed under the control of MyoD and Mef2C, and, together with miR-133 and miR-181, regulates SC fate [37]. miR-27b tunes Pax3 expression and promotes a rapid and robust entry into the myogenic program [38], whereas miR-489 is highly expressed during quiescence and is quickly downregulated during SC activation [39]. In addition, lncRNAs are also emerging as regulators of the myogenic program [40]. In particular, low or high levels of lnc-MD1 correlate with, respectively, delayed or early onset of SC specification by modulating Mef2C levels, and lnc-MD1 is strongly reduced in SCs from Duchenne muscular dystrophy (DMD) patients [41].
Besides the potential of the SC pool, multiple studies suggest that also other resident cells of different origins and not localized in the SC niche are able to transit from the interstitial compartment and to contribute to skeletal muscle plasticity [42]. SCs cannot migrate through the circulation and easily undergo senescence after ex vivo expansion, thus novel progenitors, featuring myogenic, proliferative, and migratory properties, are particularly attractive for translating somatic cell plasticity into putative treatments.
A potential source of muscle progenitors consists of vessel-associated stem cells, i.e., mesoangioblasts (MABs), which reside around the microvasculature bedewing the skeletal muscle [43]. MABs express pericytic markers, e.g., stem cell antigen 1 (Sca1) (although restricted to murine cells), NG2, Alkaline Phosphatase (AP), CD140a, and CD140b, and can be easily isolated from adult muscles of mice, dogs, and humans [44–47]. Interestingly, MABs are proliferative and multipotent, due to their capability to differentiate toward myogenic, osteogenic, chondrogenic, and adipogenic lineages [48]. MABs have been demonstrated to undergo skeletal myogenesis in vitro and in vivo. When intra-arterially injected in αSarcoglycan-null (Sgca-null) mice or in Golden Retriever dogs exhibiting DMD, MABs are able to fuse and generate sarcoglycan+ or dystrophin+ fibers, improving electrophysiological properties of injected muscles [43, 44]. Moreover, when engineered to express human minidystrophin and transplanted into scid/mdx immunodeficient dystrophic mice, DMD MABs give rise to fibers expressing a mature pattern of the transgene [45]. Based on these observations and on the suitability of MABs to systemic delivery, a phase-I/II clinical trial is currently ongoing on DMD patients (EudraCT No. 2011-000176-33).
Interstitial stem cells are another population of Pax7−/Sca1+/CD34+ cells and are characterized by expression of the stress mediator Pw1, a zinc-finger-containing protein expressed by C2C12 myoblasts but absent in fibroblasts [49]. Pw1+ interstitial cells (PICs) are self-renewing and have been identified in mouse as myogenic progenitors able to differentiate both to skeletal and smooth muscle cells in vitro [50]. Further studies will be necessary to identify the human counterpart of these cells and to evaluate their translational potential.
Recently, other stromal cells have been suggested to play a supportive role in myogenic differentiation. Fibro/adipocyte progenitors (FAPs) are as abundant as SCs and show a strong predisposition toward the generation of adipocytes and myofibroblasts. Two independent groups have isolated FAPs as CD34+/Sca1+/Lin−/CD31−/CD45−/α7integrin− or as Sca1+/CD140a+/α7integrin− populations respectively [51, 52]. In resting muscles, the interaction with intact myofibers prevents FAP differentiation into fibro-adipocytes [53]. However, muscle injury stimulates these cells to produce paracrine factors such as IL-6 and IGF-1 that positively influence myogenic differentiation [51]. In a recent study, FAPs from young mdx mice have been shown to promote in vitro SC-mediated formation of myotubes. Moreover, histone de-acetylase (HDAC) inhibitor enhances FAP ability to promote differentiation of adjacent SCs, through upregulation of the soluble factor follistatin, while inhibiting FAP adipogenic potential [54]. Because the human counterpart of Sca1 antigen is currently unknown and FAPs are isolated as Sca1+ cells in mice, future efforts should be directed towards the identification of reliable markers that can allow the isolation of FAPs from human biopsies.
Thus, enhancing the basic knowledge and the translational application of novel myogenic candidates, and their relations with SCs, will likely corroborate current regenerative strategies for the skeletal muscle, through either cell transplantation, either support of the endogenous potential.
Stem cell plasticity in the post-natal cardiac muscle
During the last 15 years, the intrinsic possibility of heart renewal has been suggested through numerous findings, including the discovery of cycling cardiomyocytes after myocardial infarction in human hearts [55] and the measurement of 14C incorporation by cardiomyocytes in humans from 1955 onwards [56]. Although controversies persist on whether the primary contribution to heart renewal in homeostatic and pathological conditions relies on pre-existing cardiomyocytes (CMs) [57] or resident progenitors [58], it is certain that the booming field has fostered the isolation of many types of resident cardiac stem cells (CSCs) from the post-natal heart, with diverse characteristics and plastic potential in vitro and in vivo. Assessment of the regenerative potential of resident CSCs, whose subset is reviewed here (Table 2; Fig. 2), will certainly help in shedding light on post-natal cardiac plasticity and on novel therapeutic strategies.
Somatic CSCs can be prospectively isolated according to specific combinations of surface antigens and culture conditions. A possible surface marker of CSCs is cKit (CD117) [59], the tyrosine kinase receptor of the stem cell factor (SCF) ligand. cKit+/Lin− CSCs form rare clusters, preferentially embedded in atria and apexes, express cardiomyogenic transcription factors, e.g., Nkx2.5, Gata4, and Mef2C, and contact the surrounding myocardium through N- and E-cadherins [60]. Once clonally expanded ex vivo and intramyocardially injected at the borders of the infarcted myocardium in rats, EGFP+ cKit+ CSCs extensively engraft in the ischemic region, differentiating into EGFP+ CMs, smooth muscle cells (SMCs) and endothelial cells (ECs) and improving ventricular functionality [61]. Similar observations of clonal plasticity in vitro and in vivo have also been reported with murine and human c-Kit (CD117)+ CSCs [62]. Moreover, a phase-1 clinical trial is currently assessing the safety and efficacy of intracoronary injection of autologous cKit+ CSCs in patients with ischemic cardiomyopathy, with preliminary beneficial effects on the left ventricle ejection fraction [63]. Interestingly, the regenerative potential of these CSCs apparently parallels the decreasing adaptation potential of the ageing heart. As compared to younger individuals, the pool of cKit+ CSCs of aged rats presents shortened telomeres, accumulation of the senescence marker p16INK4a and decreased expression levels of Igf1 and Hgf [64], ligands that promote CSC proliferation [65] and homing [66], respectively. Accordingly, in human hearts affected by acute or chronic infarcts, when compared to healthy controls, CSCs present significantly higher levels of not only commitment toward CMs, SMCs, and ECs, but also senescence and apoptosis [67].
Another surface marker to isolate putative CSCs in rodents is the Sca1 [68], a member of Ly-6 antigen family. Once isolated from the adult murine myocardium, Sca1+ CSCs are CD31+/CD38+ and Lin−/cKit−/CD4−/CD8−/CD34−/CD45−/Flk1−, and express Gata4 and Mef2C but not Nkx2.5. After intravenous delivery after ischemia/reperfusion injury, Sca1+ CSCs home at the borders of the infarcted region and differentiate into mature grafts, probably through both de novo CM production and fusion with pre-existing CMs [69]. Moreover, clonal Sca1+ CSC pools can form monolayer sheets that, once transplanted in ischemic hearts, graft in the myocardium and stimulate EC mobilization, by secreting soluble VCAM1 [70]. Interestingly, Sca1+ CSCs present features of in vitro plasticity, as they can differentiate either into beating CMs, when stimulated with oxytocin, either into osteocytes and adipocytes, in the presence of appropriate inducing conditions [71]. In contrast, intramyocardial injection of Sca1+/CD31− cells alleviates functional decline and adverse remodeling of the ischemic left ventricle, primarily triggering angiogenesis and CM function through paracrine signals, rather than directly differentiating into CMs or ECs [72].
CSCs can be also isolated according to distinctive morphological features, such as cardiospheres (CSs). CSs spontaneously arise after subculture of heart biopsies of rodents, pigs, and humans, and contain a heterogeneous mix of immature cells, partially positive for cKit, CD34, or Flk1 expression, and committed cells, presenting expression of cTnI, ANP, and MyHC. Murine CSs spontaneously beat in vitro, whereas porcine and human CSs display beating potential in co-culture with neonatal rat cardiomyocytes [73]. Moreover, CS-derived cells (CDCs) can be expanded from porcine and human CSs as cKit+/CD31+/CD34+/CD90+/CD105+ heterogeneous pool and, when injected in infarcted ventricles, differentiate into CMs and ECs, leading to increased ejection fraction [74]. Notably, long-term beneficial effects on the infarcted myocardium can also be achieved with injection of allogeneic CDCs, transiently engrafting and stimulating resident CSCs and angiogenesis [75]. Notwithstanding CS innate heterogeneity, intracoronary injection of autologous CDCs has been proven safe and partially efficacious in a phase 1 clinical trial on patients with myocardial infarction [76].
CSC pools can also be defined by specific transcription factors, such as Islet1 (Isl1) or Wilm’s tumor 1 (Wt1), although the CSC sources are then confined to transgenic animal or cellular systems. Isl1 is a homeodomain-containing transcription factor, expressed during secondary heart field specification and identifying a plastic progenitor pool, differentiating into CMs, SMCs, and ECs during murine [77] and human [78] development. After birth, Isl1 is largely repressed and its expression is confined to a rare subset (≈500 cells per rat heart) of resident CSCs, scattered within ventricular myocardium or arranged in clusters in the atria. Isl1 + CSCs strongly upregulate Nkx2.5 and Gata4 expression after isolation and differentiate in vitro into electrically competent CMs without cell fusion [79]. Wt1 is a transcription factor identifying a pool of epicardial progenitors, which contribute to the murine fetal cardiomyogenesis [80] and, in the presence of thymosin β4, form vascular precursors [81]. Intriguingly, pre-treatment of murine adult hearts with thymosin β4 reactivates Wt1 + resident CSCs, expressing Sca1, Isl1, and Nkx2.5, differentiating into electrically coupled CMs in the ischemic myocardium and ameliorating functional outcome after infarction [82].
An alternative source of cardiac regeneration potential relies on cardiac pericytes and, particularly, cardiac mesoangioblasts (cMABs). cMABs can be isolated from murine, canine, and human cardiac explants and display pericytic markers, e.g., NG2 and AP, in combination with cardiomyogenic transcription factors, e.g., Nkx2.5 and Gata4 [83]. Low-passage murine cMABs differentiate in vitro into beating cardiomyocytes, presenting sarcomeric structures and Cx43 junctions and, after intraventricular delivery in ischemic hearts, mainly home at the periphery of the necrotic area and differentiate into CMs, participating in the myocardial regeneration [84]. Remarkably, when isolated from samples of canine [85] and human [83] cardiomyopathic hearts, cMABs present impairment of several markers of proliferation and plasticity. Furthermore, aorta-derived MABs (ADMs) present plasticity toward mesodermal and ectodermal derivatives. When injected intramyocardially, ADMs engraft the myocardium of dystrophic mice, differentiating into CMs and ECs and preventing the onset of dilated cardiomyopathy [86], whereas, under appropriate inducing conditions, ADMs can also transdifferentiate in vitro and in vivo toward myelinating glial cells [87]. Although prone to senescence and less efficient in vivo than other resident CSC types, cardiomyogenic MABs constitute an interesting reservoir of cell plasticity, given their relative abundance around the rich microvasculature network bedewing the cardiac muscle.
Non-resident stem cells for skeletal and cardiac muscle regeneration
Besides the intrinsic stem cell pools, other non-resident adult stem cells have been reported to positively contribute to the regeneration of skeletal and cardiac muscle. Because already extensively treated in dedicated reviews [88, 89] and considering that this review preferentially deals with the resident populations, we will briefly report here several interesting studies involving progenitors isolated from the bone marrow (BM) and the peripheral blood (Fig. 2). Because they are relatively easy to isolate and expand, BM- or blood-derived progenitors are still potentially attractive for regenerative studies of the post-natal muscles.
Fig. 2.
Schematic representation of resident (within upper colored lobes) and non-resident stem cells for post-natal plasticity in skeletal and cardiac muscles, as reviewed in the text. Within the lower lobe, resident stem cells undergoing lineage switch are reported
Only 0.0001–0.001 % of nucleated cells in adult BM are considered to be mesenchymal stem cells (MSCs) [90, 91]. Identified by the expression of CD73 and CD105, MSCs do not express other hematopoietic or endothelial markers such as CD14, CD31, CD34, CD45. Moreover, MSCs are CD29+/CD44+/CD71+/CD90+/CD106+/CD166+ [92, 93]. BM-derived MSCs grow as adherent cells in vitro and differentiate under defined conditions into various tissues, including bone, cartilage, muscle, marrow stroma, tendon, ligament, adipous, and other connective tissues [94]. Since 20 years, the myogenic potential of MSCs and other BM-derived progenitors has been investigated. Progenitors from the BM are recruited and participate in muscle regeneration, and donor nuclei are traceable after many years at a very low frequency [95]. Multipotency and ease of isolation render adult MSCs an attractive candidate for stem cell therapy and several circulating BM-derived stem cells can participate in skeletal muscle regeneration, although further studies will be useful to pinpoint the cues eliciting recruitment and transdifferentiation [96–98]. In contrast, other reports suggest that MSC myogenic capability is per se scarce, and that beneficial effects are limited to local cell recruitment and paracrine effects [99–101]. Nevertheless, tuning key molecular pathways could in principle enhance MSC intrinsic commitment toward skeletal muscle, as reported with Notch signaling overactivation [102].
Regarding the regeneration of the cardiac muscle, BM-derived cKit+/Lin− stem cells show dramatic engraftment in the infarcted myocardium and robust differentiation into CMs, SMCs, and ECs, when intramyocardially administered after coronary ligation [103]. However, systemic intravenous injection of Sca1+/cKit+ cells, isolated as BM side population (BM-SP) according to Hoechst 33342 dye efflux, resulted in low rates of engraftment and negligible levels of regeneration in the chronically damaged striated muscle of dystrophic δSarcoglycan-null (Sgcd-null) mice [104].
Endothelial progenitor cells (EPCs) from the peripheral circulation constitute another attractive source of non-resident cells to favor muscle repair, because they are easily isolatable and highly angiogenic. CD34+ circulating EPCs promote extensive neovascularization of ischemic hind-limb muscles [105] and myocardium [106], after intramuscular and intravenous injection, respectively. Interestingly, when co-cultured with neonatal rat cardiomyocytes, human EPCs transdifferentiate in vitro toward cardiomyocyte-like cells, exhibiting calcium transients and functional gap junctions, without cell fusion [107].
Thus, a more refined knowledge of intrinsic mechanisms and extrinsic manipulation of non-resident somatic cells is still required to better coax them in acquiring myogenic commitment or, intriguingly, in supporting resident myogenic progenitors.
Is the switch between the two myogenic lineages possible?
Both skeletal and cardiac muscles originate from embryonic mesoderm and, during adulthood, present intrinsic regenerative capacity. However, while the regenerative machinery of the skeletal muscle is efficient, extensive damage in the heart results in scar tissue formation, compromising cardiac function. Despite profound differences in regenerative potential and specific stem cell properties, the two types of striated muscle share many molecular, structural, and functional features. In this perspective, the question whether a crossover between the two lineages is possible in vivo gathers challenging answers, particularly in terms of post-natal cell plasticity.
Hereafter some cell lines able to present these characteristics are reported (Table 3; Fig. 2). A paradigmatic case of lineage crossover is constituted by the cranial paraxial mesoderm (CPM) in chick embryos. The neural tube inhibits cardiomyogenesis in CPM by releasing Wnt agonists, whereas ectopic secretion of Wnt antagonists or Bmp4 conversely promote migration of CPM progenitors toward the developing heart [108]. Accordingly, CPM progenitors can also be traced in their migration and participation to the cardiac outflow tract [109]. In addition, in vitro CPM explants express MyoD, Myf5, and Myogenin, markers of skeletal myogenesis, and, in the presence of Bmp4, also activate the expression of Nkx2.5, Gata4, Gata5, Gata6, and Isl1, markers of early cardiomyogenesis [109].
Table 3.
Cell progenitors exhibiting potential lineage switch between skeletal and cardiac muscle
| Cell type | Species | Surface antigens | Lineage markers | In vivo plasticity | References | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| SPOCs | Mouse | Sca1 | cKit, CD34, CD45 | Nkx2.5, Gata4 | CMs | [110] |
| Sk-34 cells | Mouse | CD34 | CD45 | NA | Skeletal fibers, ECs, Schwann cells | [112] |
| Sk-34 cells | Mouse | CD34 | CD45 | Pax7, MyoD, Myf5, Isl1, Gata4, Mef2C, Hand2 | CMs, ECs | [113] |
| Sgcb-null cMABs | Mouse | AP, NG2, Sca1, cKit, CD31, CD34, CD44, CD140b | CD13, CD45, CD56 | Pax3, MyoD, Myf5, Myogenin, Isl1, Nkx2.5, Gata4, Mef2A | Skeletal fibers | [115] |
Each entry relates to one reference and the entry order is referred to the review
NA information not available in the cited reference, SPOCs skeletal-based precursors of cardiomyocytes, Sk-34 cells skeletal-derived CD34+ myoendothelial cells, Sgcb-null cMABs cardiac mesoangioblasts isolated from βSarcoglycan-null (Sgcb-null) mice, CMs cardiomyocytes, ECs endothelial cells
Intriguingly, cell pools switching from skeletal to cardiac lineage have also been isolated from adult murine skeletal muscles. On one hand, skeletal-based precursors of cardiomyocytes (SPOCs) are isolated as round, floating CD34−/CD45−/cKit− cells and express markers of cardiac, but not skeletal, myogenesis, when cultured in the presence of EGF and FGF. SPOCs differentiate in vitro into beating cardiomyocytes, derived from the Sca1− subfraction, and, after intravenous delivery, engraft in the ischemic ventricle and partially differentiate into CMs at the peripheral region of the infarct [110]. On the other hand, skeletal-derived CD34+ (Sk-34) myoendothelial cells can be sorted from the interstitial tissue as CD34+/CD45−, are Sca1+/cKit−/CD31− and express, once in culture, skeletal myogenic markers [111]. After intramuscular injection into severely damaged skeletal muscles, freshly isolated Sk-34 cells extensively engraft and generate myogenic, endothelial, and Schwann cells, thus exhibiting broad in vivo plasticity toward mesodermal and ectodermal lineages [112]. Strikingly, Sk-34 cells undergo also cardiomyogenic transdifferentiation in co-culture with fetal cardiomyocytes in vitro and, once intramyocardially injected, participate in the regeneration of infarcted rat hearts, with significant improvement of left ventricle functionality [113].
Furthermore, post-natal stem cells crossing from cardiac to skeletal lineage have also been reported, shedding new light on the cardiomyopathic progression in a murine model of muscular dystrophy [114]. When isolated from βSarcoglycan-null (Sgcb-null) dystrophic mice, in fact, cMABs co-express cardiac and skeletal myogenic transcriptions factors. Moreover, cMABs spontaneously differentiate into skeletal myotubes in vitro and arrhythmogenic skeletal muscle patches in vivo, after intramyocardial injection into infarcted hearts. The robust lineage shift relies on downregulation, due to calcium leakage by the plasma membrane and knockout of the first Sgcb intron, respectively, of miRNAs miR-669a/q, normally repressing MyoD translation. Reintroduction of miR-669a, in fact, partially rescues the cardiomyogenic commitment of Sgcb-null both in vitro and in vivo [115] and results in alleviation of the cardiomyopathy in the long term.
However, translation of basic research on lineage plasticity into human patients is still facing prominent hurdles. After the first promising reports of feasibility and efficacy of SC therapy for cardiac repair in freeze-injured hearts of rats [116] and rabbits [117], subsequent clinical trials have yielded contrasting results. In patients undergoing coronary artery bypass graft, injection of autologous SCs (approximately 65 % CD56+) in and around the post-infarction scar tissue increases regional contractility and viability of the scarred myocardium in both the first clinical case [118] and the first 1-year follow-up [119]. Nevertheless, when tested in a multi-centered randomized trial, autologous SC therapy fails to induce significant beneficial effects on regional or global ventricular function and increases the number of early post-operative arrhythmogenic events, as compared to placebo [120].
However, recent observations in infarcted rats suggest that application of SC sheets on the epicardium of damaged ventricles alleviates hypertrophy and ameliorates ventricular function, avoiding the events of arrhythmia and tachycardia observed after injection of SC suspension [121]. Moreover, when engineered to secrete an artificial angiogenic peptide, SC sheets further improve also novel vessel formation around the infarct area [122].
Therefore, new evidences suggest the need for further research in this provocative field, learning from and potentially bypassing hurdles and failures of past attempts.
Conclusions
In conclusion, many theoretical as well as applicative questions about the cell plasticity in post-natal skeletal and cardiac muscles still remain open.
With regards to the adult mammalian skeletal muscle, many types or subtypes of resident stem cells have been isolated and characterized. However, further translational studies are required to address the boundaries of the intrinsic plastic potential of each cell type. Moreover, it will be particularly intriguing to deepen our knowledge about the epigenetic signatures potentially regulating intrinsic fate choices and to define the patterns of cellular crosstalk among the different pools. This will be useful to enhance applicability of regenerative medicine to the skeletal muscle, through not only cell-based strategies but also stimulation of the endogenous potential.
Similarly, numerous CSC pools with different characteristics have been isolated from the adult cardiac muscle of mammals according to often-incomparable procedures of isolation and handling. Additionally, refined details about epigenetic control on CSC potency and commitment are still lacking. Moreover, it is still an open question whether the different CSC types derive from actual distinguishable pools in vivo or represent different states of endogenous CSC activation or ex vivo culture. Results from the first clinical trials and more refined translational studies will probably help in shedding light on this controversial issue.
Finally, with regards to the lineage switch between skeletal and cardiac myogenesis, the path is still long before achieving translational relevance, particularly within adult stem cell pools. Nonetheless, advancing our knowledge of myogenic lineage plasticity still constitutes an intriguing perspective not only for regenerative medicine but also for drug screening and disease modeling systems. In this view, fundamental insights will be gained by combining epigenetic, transcriptional, and signaling studies at the cellular or tissue level. Converting these complex data networks into refined in vitro/in vivo approaches will then constitute the necessary challenge to enhance the myogenic potential of adult stem cells and other post-natal reservoirs of potency, such as induced pluripotent stem cells.
Acknowledgments
We ask forgiveness of the authors whose work was not cited here due to space constraints. The Translational Cardiomyology laboratory is supported by CARE-MI FP7, AFM, CARIPLO, FWO, GOA, UIAP and OT grants. We are particularly grateful to Giulio Cossu, Jan Deprest, Paul Holvoet, Danny Huylebroeck, Frank Luyten, Karin Sipido, and Catherine Verfaillie for critical discussions. The authors would also like to thank Christina Vochten and Vicky Raets for professional secretarial service and Paolo Luban and Rondoufonds voor Duchenne Onderzoek for kind donations.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
D. Costamagna and M. Quattrocelli contributed equally to this work.
References
- 1.Biressi S, Molinaro M, Cossu G. Cellular heterogeneity during vertebrate skeletal muscle development. Dev Biol. 2007;308:281–293. doi: 10.1016/j.ydbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Aulehla A, Pourquie O. On periodicity and directionality of somitogenesis. Anat Embryol (Berl) 2006;211(Suppl 1):3–8. doi: 10.1007/s00429-006-0124-y. [DOI] [PubMed] [Google Scholar]
- 3.Kahane N, Cinnamon Y, Bachelet I, Kalcheim C. The third wave of myotome colonization by mitotically competent progenitors: regulating the balance between differentiation and proliferation during muscle development. Development. 2001;128:2187–2198. doi: 10.1242/dev.128.12.2187. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 5.Bober E, Franz T, Arnold HH, Gruss P, Tremblay P. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994;120:603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- 6.Tremblay P, Gruss P. Pax: genes for mice and men. Pharmacol Ther. 1994;61:205–226. doi: 10.1016/0163-7258(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 7.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 8.Edmondson DG, Olson EN. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 9.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu Rev Cell Dev Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 10.Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- 11.Chan SS, Shi X, Toyama A, Arpke RW, Dandapat A, Iacovino M, Kang J, Le G, Hagen HR, Garry DJ, et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell. 2013;12:587–601. doi: 10.1016/j.stem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morin S, Charron F, Robitaille L, Nemer M. GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 2000;19:2046–2055. doi: 10.1093/emboj/19.9.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol Cell Biol. 2000;20:7550–7558. doi: 10.1128/mcb.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh TK, Song FF, Packham EA, Buxton S, Robinson TE, Ronksley J, Self T, Bonser AJ, Brook JD. Physical interaction between TBX5 and MEF2C is required for early heart development. Mol Cell Biol. 2009;8:2205–2218. doi: 10.1128/MCB.01923-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincentz JW, Barnes RM, Firulli AB. Hand factors as regulators of cardiac morphogenesis and implications for congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2011;91:485–494. doi: 10.1002/bdra.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedesco FS, Dellavalle A, Diaz-Manera J, Messina G, Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest. 2010;120:11–19. doi: 10.1172/JCI40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 22.Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–196. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 23.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono Y, Calhabeu F, Morgan JE, Katagiri T, Amthor H, Zammit PS. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011;18:222–234. doi: 10.1038/cdd.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun T, Rudnicki MA, Arnold HH, Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992;71:369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- 27.Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Fujii J, Phillips MS, Chen HS, Karpati G, Yee WC, Schrank B, Cornblath DR, Boylan KB, MacLennan DH. Characterization of cDNA and genomic DNA encoding SERCA1, the Ca(2+)-ATPase of human fast-twitch skeletal muscle sarcoplasmic reticulum, and its elimination as a candidate gene for Brody disease. Genomics. 1995;30:415–424. doi: 10.1006/geno.1995.1259. [DOI] [PubMed] [Google Scholar]
- 29.Kassar-Duchossoy L, Gayraud-Morel B, Gomes D, Rocancourt D, Buckingham M, Shinin V, Tajbakhsh S. Mrf4 determines skeletal muscle identity in Myf5: Myod double-mutant mice. Nature. 2004;431:466–471. doi: 10.1038/nature02876. [DOI] [PubMed] [Google Scholar]
- 30.Valdez MR, Richardson JA, Klein WH, Olson EN. Failure of Myf5 to support myogenic differentiation without myogenin, MyoD, and MRF4. Dev Biol. 2000;219:287–298. doi: 10.1006/dbio.2000.9621. [DOI] [PubMed] [Google Scholar]
- 31.Parker MH, von Maltzahn J, Bakkar N, Al-Joubori B, Ishibashi J, Guttridge D, Rudnicki MA. MyoD-dependent regulation of NF-kappaB activity couples cell-cycle withdrawal to myogenic differentiation. Skelet Muscle. 2012;2:6. doi: 10.1186/2044-5040-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Imai S, Johnson FB, Marciniak RA, McVey M, Park PU, Guarente L. Sir2: an NAD-dependent histone deacetylase that connects chromatin silencing, metabolism, and aging. Cold Spring Harb Symp Quant Biol. 2000;65:297–302. doi: 10.1101/sqb.2000.65.297. [DOI] [PubMed] [Google Scholar]
- 33.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 34.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathbone CR, Booth FW, Lees SJ. Sirt1 increases skeletal muscle precursor cell proliferation. Eur J Cell Biol. 2009;88:35–44. doi: 10.1016/j.ejcb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HK, Lee YS, Sivaprasad U, Malhotra A, Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol. 2006;174:677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, Conway SJ, Buckingham M. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci USA. 2009;106:13383–13387. doi: 10.1073/pnas.0900210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482:524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Leeuwen S, Mikkers H. Long non-coding RNAs: guardians of development. Differentiation. 2010;80:175–183. doi: 10.1016/j.diff.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 41.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, Tramontano A, Bozzoni I. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quattrocelli M, Cassano M, Crippa S, Perini I, Sampaolesi M. Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ. 2010;17:1222–1229. doi: 10.1038/cdd.2009.160. [DOI] [PubMed] [Google Scholar]
- 43.Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D’Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 44.Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 45.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 46.Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 47.Tonlorenzi R, Dellavalle A, Schnapp E, Cossu G, Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. Curr Protoc Stem Cell Biol. 2007;Chapter 2:Unit 2B 1. doi: 10.1002/9780470151808.sc02b01s3. [DOI] [PubMed] [Google Scholar]
- 48.Quattrocelli M, Palazzolo G, Perini I, Crippa S, Cassano M, Sampaolesi M. Mouse and human mesoangioblasts: isolation and characterization from adult skeletal muscles. Methods Mol Biol. 2012;798:65–76. doi: 10.1007/978-1-61779-343-1_4. [DOI] [PubMed] [Google Scholar]
- 49.Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- 51.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, Liu Y, Lai X, Kuang S. Intramuscular adipose is derived from a non-Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol. 2012;361:27–38. doi: 10.1016/j.ydbio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, et al. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO Mol Med. 2013;5:626–639. doi: 10.1002/emmm.201202096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, Nadal-Ginard B, Silvestri F, Leri A, Beltrami CA, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–1757. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 56.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. J Clin Invest. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci USA. 2006;103:9226–9231. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 62.Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci USA. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126(11 Suppl 1):S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, et al. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 65.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 66.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh H, Chi X, Bradfute SB, Mishina Y, Pocius J, Michael LH, Behringer RR, Schwartz RJ, Entman ML, Schneider MD. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci. 2004;1015:182–189. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- 69.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, et al. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]
- 73.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 74.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 75.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, et al. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 78.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, Roberts DJ, Huang PL, Domian IJ, Chien KR. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 79.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- 82.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galvez BG, Covarello D, Tolorenzi R, Brunelli S, Dellavalle A, Crippa S, Mohammed SA, Scialla L, Cuccovillo I, Molla F, et al. Human cardiac mesoangioblasts isolated from hypertrophic cardiomyopathies are greatly reduced in proliferation and differentiation potency. Cardiovasc Res. 2009;83:707–716. doi: 10.1093/cvr/cvp159. [DOI] [PubMed] [Google Scholar]
- 84.Galvez BG, Sampaolesi M, Barbuti A, Crespi A, Covarello D, Brunelli S, Dellavalle A, Crippa S, Balconi G, Cuccovillo I, et al. Cardiac mesoangioblasts are committed, self-renewable progenitors, associated with small vessels of juvenile mouse ventricle. Cell Death Differ. 2008;15:1417–1428. doi: 10.1038/cdd.2008.75. [DOI] [PubMed] [Google Scholar]
- 85.Casssano M, Berardi E, Crippa S, Toelen J, Barthelemy I, Micheletti R, Chuah M, Vandendriessche T, Debyser Z, Blot S, et al. Alteration of cardiac progenitor cell potency in GRMD dogs. Cell Transpl. 2012;21:1945–1967. doi: 10.3727/096368912X638919. [DOI] [PubMed] [Google Scholar]
- 86.Chun JL, O’Brien R, Song MH, Wondrasch BF, Berry SE. Injection of vessel-derived stem cells prevents dilated cardiomyopathy and promotes angiogenesis and endogenous cardiac stem cell proliferation in mdx/utrn−/− but not aged mdx mouse models for duchenne muscular dystrophy. Stem Cells Transl Med. 2013;2:68–80. doi: 10.5966/sctm.2012-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Kamath A, Frye J, Iwamoto GA, Chun JL, Berry SE. Aorta-derived mesoangioblasts differentiate into the oligodendrocytes by inhibition of the rho kinase signaling pathway. Stem Cells Dev. 2012;21:1069–1089. doi: 10.1089/scd.2011.0124. [DOI] [PubMed] [Google Scholar]
- 88.Mendez-Ferrer S, Ellison GM, Torella D, Nadal-Ginard B. Resident progenitors and bone marrow stem cells in myocardial renewal and repair. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S83–S89. doi: 10.1038/ncpcardio0415. [DOI] [PubMed] [Google Scholar]
- 89.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–3493. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- 90.Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995;18:1417–1426. doi: 10.1002/mus.880181212. [DOI] [PubMed] [Google Scholar]
- 91.Gridley DS, Pecaut MJ. Whole-body irradiation and long-term modification of bone marrow-derived cell populations by low- and high-LET radiation. In Vivo. 2006;20:781–789. [PubMed] [Google Scholar]
- 92.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 93.Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 94.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 95.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 96.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–1345. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 97.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 98.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 99.Partridge T. Versatility and commitment in muscle. J Physiol. 2005;562:646. doi: 10.1113/jphysiol.2004.080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wernig G, Janzen V, Schafer R, Zweyer M, Knauf U, Hoegemeier O, Mundegar RR, Garbe S, Stier S, Franz T, et al. The vast majority of bone-marrow-derived cells integrated into mdx muscle fibers are silent despite long-term engraftment. Proc Natl Acad Sci USA. 2005;102:11852–11857. doi: 10.1073/pnas.0502507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salah-Mohellibi N, Millet G, Andre-Schmutz I, Desforges B, Olaso R, Roblot N, Courageot S, Bensimon G, Cavazzana-Calvo M, Melki J. Bone marrow transplantation attenuates the myopathic phenotype of a muscular mouse model of spinal muscular atrophy. Stem Cells. 2006;24:2723–2732. doi: 10.1634/stemcells.2006-0170. [DOI] [PubMed] [Google Scholar]
- 102.Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 103.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 104.Lapidos KA, Chen YE, Earley JU, Heydemann A, Huber JM, Chien M, Ma A, McNally EM. Transplanted hematopoietic stem cells demonstrate impaired sarcoglycan expression after engraftment into cardiac and skeletal muscle. J Clin Invest. 2004;114:1577–1585. doi: 10.1172/JCI23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kudo FA, Nishibe T, Nishibe M, Yasuda K. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int Angiol. 2003;22:344–348. [PubMed] [Google Scholar]
- 106.Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 107.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 108.Tzahor E, Lassar AB. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001;15:255–260. doi: 10.1101/gad.871501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133:1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- 110.Winitsky SO, Gopal TV, Hassanzadeh S, Takahashi H, Gryder D, Rogawski MA, Takeda K, Yu ZX, Xu YH, Epstein ND. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 2005;3:e87. doi: 10.1371/journal.pbio.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tamaki T, Uchiyama Y, Okada Y, Ishikawa T, Sato M, Akatsuka A, Asahara T. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation. 2005;112:2857–2866. doi: 10.1161/CIRCULATIONAHA.105.554832. [DOI] [PubMed] [Google Scholar]
- 113.Tamaki T, Akatsuka A, Okada Y, Uchiyama Y, Tono K, Wada M, Hoshi A, Iwaguro H, Iwasaki H, Oyamada A, et al. Cardiomyocyte formation by skeletal muscle-derived multi-myogenic stem cells after transplantation into infarcted myocardium. PLoS One. 2008;3:e1789. doi: 10.1371/journal.pone.0001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crippa S, Cassano M, Sampaolesi M. Role of miRNAs in muscle stem cell biology: proliferation, differentiation and death. Curr Pharm Des. 2012;18:1718–1729. doi: 10.2174/138161212799859620. [DOI] [PubMed] [Google Scholar]
- 115.Crippa S, Cassano M, Messina G, Galli D, Galvez BG, Curk T, Altomare C, Ronzoni F, Toelen J, Gijsbers R, et al. miR669a and miR669q prevent skeletal muscle differentiation in postnatal cardiac progenitors. J Cell Biol. 2011;193:1197–1212. doi: 10.1083/jcb.201011099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 118.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 119.Gavira JJ, Herreros J, Perez A, Garcia-Velloso MJ, Barba J, Martin-Herrero F, Canizo C, Martin-Arnau A, Marti-Climent JM, Hernandez M, et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J Thorac Cardiovasc Surg. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 120.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 121.Narita T, Shintani Y, Ikebe C, Kaneko M, Harada N, Tshuma N, Takahashi K, Campbell NG, Coppen SR, Yashiro K, et al. The use of cell-sheet technique eliminates arrhythmogenicity of skeletal myoblast-based therapy to the heart with enhanced therapeutic effects. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 122.Uchinaka A, Kawaguchi N, Hamada Y, Mori S, Miyagawa S, Saito A, Sawa Y, Matsuura N. Transplantation of myoblast sheets that secrete the novel peptide SVVYGLR improves cardiac function in failing hearts. Cardiovasc Res. 2013 doi: 10.1093/cvr/cvt088. [DOI] [PubMed] [Google Scholar]