Fig. 3.
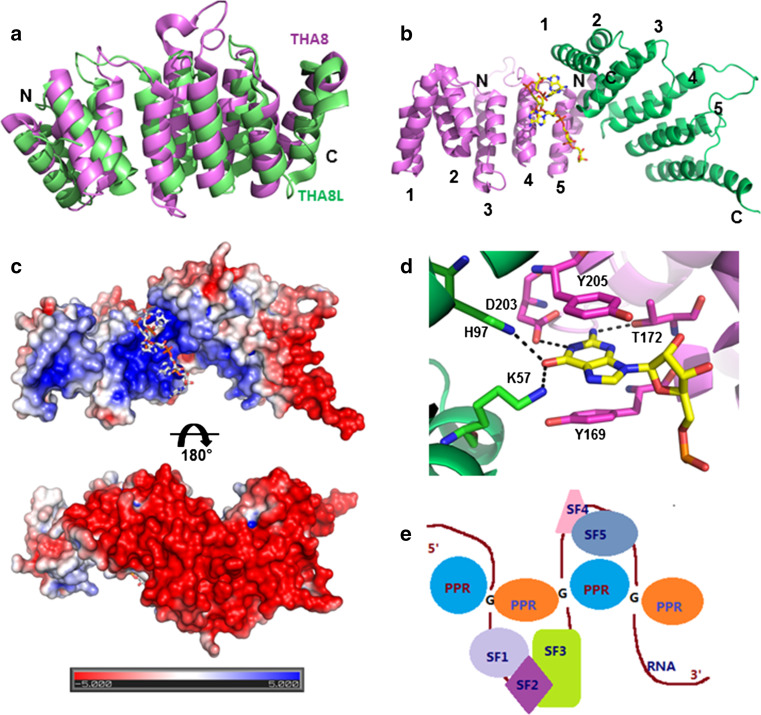
The THA8 homolog recognizes target RNAs through formation of a dimer or oligomer. a Superposition of apo-THA8 (magenta, PDB accession code: 4ME2) and THA8L (green, PDB code: 4LEU) structures in cartoon representation. b The structure of THA8 in complex with a 13-nucleotide Zm-4 RNA shown in cartoon representation, with the two monomers colored in green and magenta (PDB accession codes: 4N2Q). The bound Zm-4 RNA fragment (AGAAA) is shown as stick model at the dimer interface. c Surface charge distribution of the two different sides of the THA8 dimer. The bound RNA fragment is shown as stick model. d Close-up view of the THA8-dimer interactions with the G nucleotide of the AGAAA motif. The carbon atoms are colored in green and magenta for two monomers that interact with Zm4 RNA. Residues that interact with RNA are shown in stick representation, using the same color as the PPR repeat to which they belong. The G nucleotide is shown in stick representation, with carbons in white, nitrogens in blue, and oxygens in red. Hydrogen-bond interactions are indicated by black dashed lines. e A model for the regulation of RNA recognition by short PPR proteins. The single-stranded pre-mRNA induces the oligomerization of PPR proteins, thus bringing several discontinuous regions of RNA into proximity to be recognized by other splicing factors to facilitate the alternative splicing of introns. PPR proteins are presented as blue and orange ovals; SF splicing factors that are recruited by mRNA are colored individually, G G nucleotides that are responsible for RNA recognition