Fig. 11.
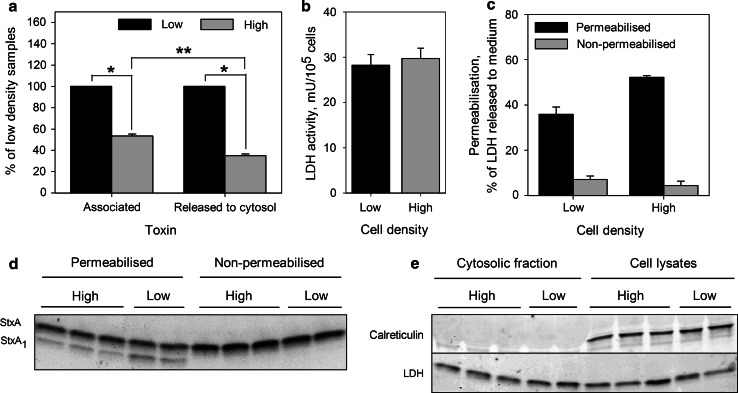
Release of StxA1 into the cytosol is reduced in high density HeLa cells. Low and high density HeLa cells were treated with 10 ng/ml 125I-Stx1-mut for 5 h at 37 °C. Then, cells were washed and incubated with fresh HEPES-buffered medium for 30 min at 37 °C to reduce the amount of surface exposed Stx. Finally, 1 mM NEM was added to cells for 5 min at 37 °C. Cells were permeabilized with activated Streptolysin O, and then incubated with transport medium for 30 min on ice to allow the cytosol to leak out (cytosolic fraction). Transport medium was then collected, and the cells lysed. The toxin was immunoprecipitated from cytosolic fraction and then separated by non-reducing SDS-PAGE. a The amount of StxA1 released to the cytosol was quantified from the autoradiogram, and adjusted according to permeabilization efficiency and cell number of each sample. To determine total cell-associated toxin, radioactivity was measured in cell lysates from non-permeabilized samples, and normalized to cell number. Finally, results were normalized to low density samples and plotted as mean value + SEM, n = 3, *p < 0.001, **p < 0.05 Student’s t test. b LDH activity per cell in low and high density samples; representative graph from one of three experiments. Error bars show the difference between the mean value and the two parallels. c The efficiency of cell permeabilization calculated as percentage of total LDH which was released to the medium; representative graph from one of three experiments. Error bars show the difference between the mean value and the two parallels. d A representative autoradiogram of immunoprecipitated toxin from the cytosolic fractions. e One-tenth of cell lysates and cytosolic fractions were precipitated by TCA, separated by reducing SDS-PAGE and blotted to PDF membrane. A representative western blot of ER protein calreticulin and LDH levels in the cytosolic fractions and cell lysates is shown