Fig. 1.
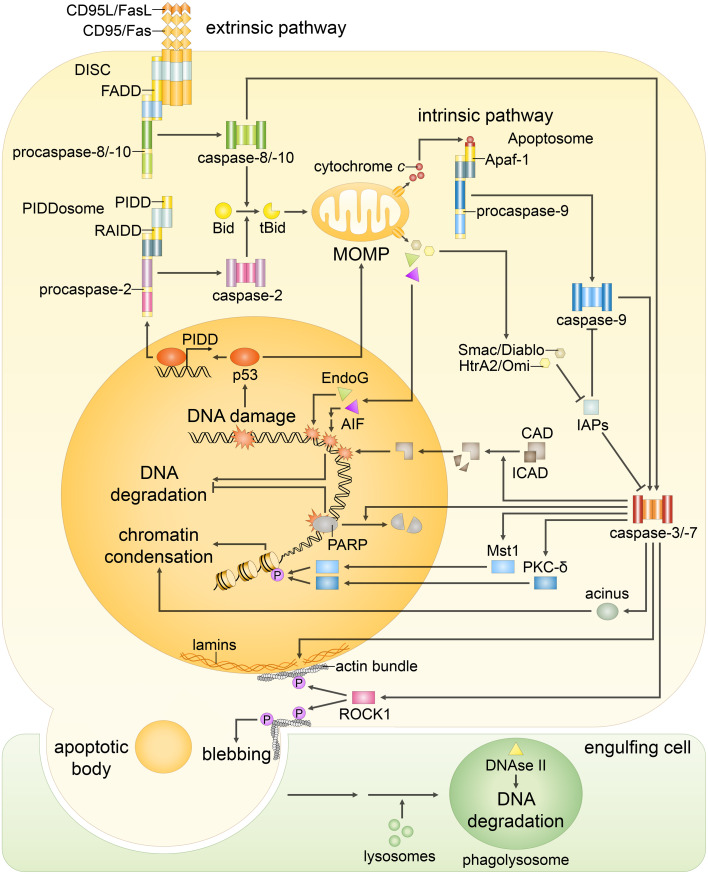
Both extrinsic and intrinsic apoptotic signaling pathways result in the same changes in the nuclear morphology. The extrinsic pathway begins with the stimulation of the death receptors by their cognate ligands that results in the assembly of initiator caspase-8/-10 activation platforms, such as death-inducing signaling complex (DISC). Caspase-8/-10 activation is followed by the cleavage of effector caspase-3, -6 and -7 and, in some cases, processing of Bid: Truncated Bid (tBid) subsequently leads to outer mitochondrial membrane permeabilization (MOMP), thereby, engaging the intrinsic pathway. The major trigger of the intrinsic pathway is DNA damage, following which MOMP is induced via several transcriptional and non-transcriptional mechanisms. MOMP results in the efflux of cytochrome c, which engages the apoptosome formation required for the activation of caspase-9. The mitochondrial proteins Smac/DIABLO and HtrA2/Omi promote apoptosis by neutralizing inhibitors of apoptosis proteins (IAPs), thus reversing their grip on caspase-3, -7, -9. In addition, in response to DNA damage formation of PIDDosome, a platform for caspase-2 activation, might be engaged. Initiator caspase-2 and -9, as well as -8 and -10, propagate the apoptotic signal by direct cleavage of the effector caspases. Effector caspases coordinate the dismantling of the nucleus. Caspase-mediated cleavage of poly(ADP-ribose) polymerase (PARP) prevents DNA repair and facilitates access of nucleases to the chromatin. Then, cleavage of inhibitor of caspase-activated DNase (ICAD) releases CAD, which enters the nucleus and catalyzes DNA degradation. Meanwhile, DNA fragmentation is also promoted in a caspase-independent manner by endonuclease G (EndoG) and apoptosis-inducing factor (AIF), which are released upon MOMP. Caspase-dependent activation of Mst1, PKC-δ and acinus stimulates chromatin condensation. Caspase-mediated cleavage of nuclear lamins contributes to nuclear fragmentation, whereas the proteolysis of the Rho effector ROCK1 results in contraction of the actin cytoskeleton that promotes breakdown of the nuclear envelope, as well as plasma membrane blebbing. Eventually, the cell collapses into apoptotic bodies, which are rapidly engulfed by phagocytic cells. Subsequently, DNA fragments, which were kept within apoptotic bodies, are digested by phagocytic DNase II