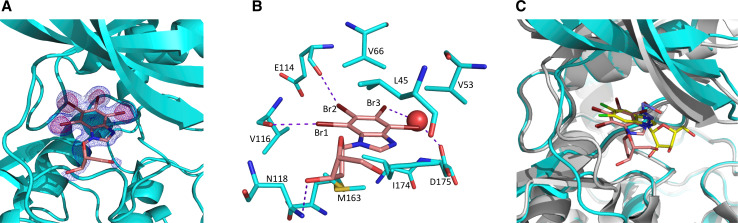
Fig. 2.
a CK2α (cyan) with bound TDB (salmon). 2Fo-Fc map contoured at 1σ is shown in blue, anomalous map contoured at 5σ is shown in pink. b TDB binds to CK2α ATP pocket. The interacting residues are shown as sticks and the bridging water molecule as a sphere. Halogen and hydrogen bonds are shown as dotted lines. c TDB binding to CK2α in orientation II compared to K68 (violet) and DRB (yellow) in orientation I. The deoxyribofuranosyl moiety of TDB is closer to the hinge region compared to the DRB ribofuranosyl group and not compatible with the closed hinge conformation observed in the DRB-bound CK2α (grey)