Table 1.
IC50 (μM) of selected compounds against CK2 and PIM-1; results are means of experiments run in triplicate, with the SEM never exceeding 10 %
| Compound | Structure | Name | CK2 (20 μM ATP) | PIM1 (100 μM ATP) |
|---|---|---|---|---|
| K156 |

|
1-(β-d-2′-deoxyribofuranosyl)-4,5,6,7-tetrachloro-1H-benzimidazole | 0.71 | 2.17 |
| K157 |

|
1-(β-d-2′-deoxyribofuranosyl)-4,7-diiodio,5,6-dichloro-1H-benzimidazole | 0.31 | 1.08 |
| K163 |

|
1-(β-d-2′-deoxyribofuranosyl)-4,7-dibromo,5,6-dichloro-1H-benzimidazole | 0.31 | 1.8 |
| TDB (K164) |
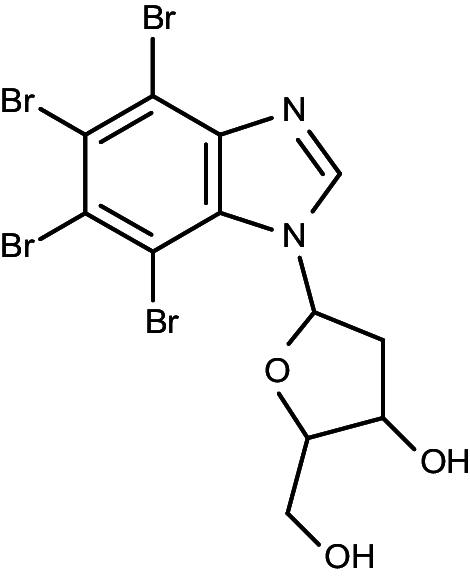
|
1-(β-d-2′-deoxyribofuranosyl)-4,5,6,7-tetrabromo-1H-benzimidazole | 0.032 | 0.086 |
| K165 |
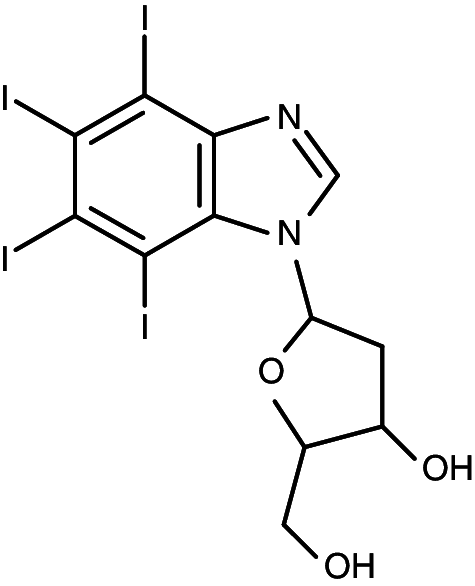
|
1-(β-d-2′-deoxyribofuranosyl)-4,5,6,7-tetraiodo-1H-benzimidazole | 0.024 | 0.43 |
| K170 |

|
1-(β-d-2′-deoxyribofuranosyl)- 5,6-dichloro-1H-benzimidazole | >40 | >40 |
| K171 |
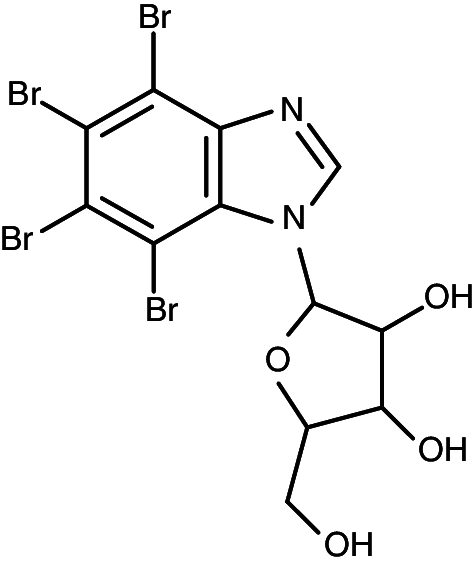
|
1-(β-d-2′-ribofuranosyl)-4,5,6,7-tetrabromo-1H-benzimidazole | 0.20 | 0.68 |
| DRB |
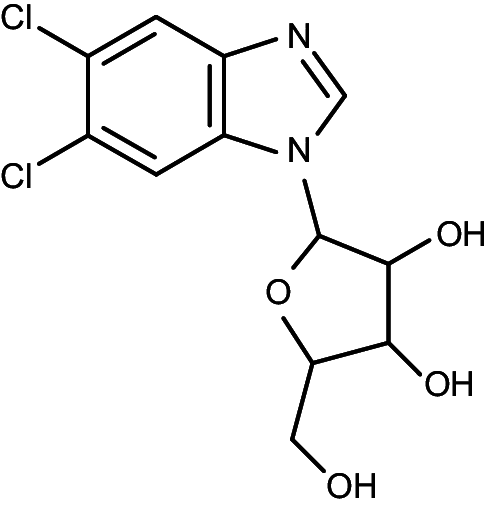
|
5,6-dichloro-1-(β-d-ribofuranosyl)benzimidazole | 38 | >40 |
| TBI (K17) |
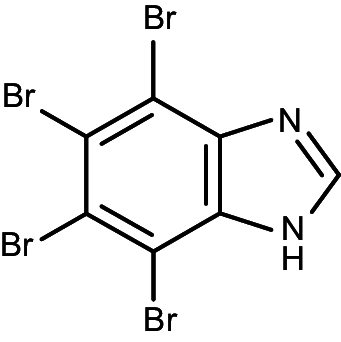
|
4,5,6,7-tetrabromo-1H-benzimidazole | 0.38 | 0.24 |
Synthesis of compounds is described in supplementary data
