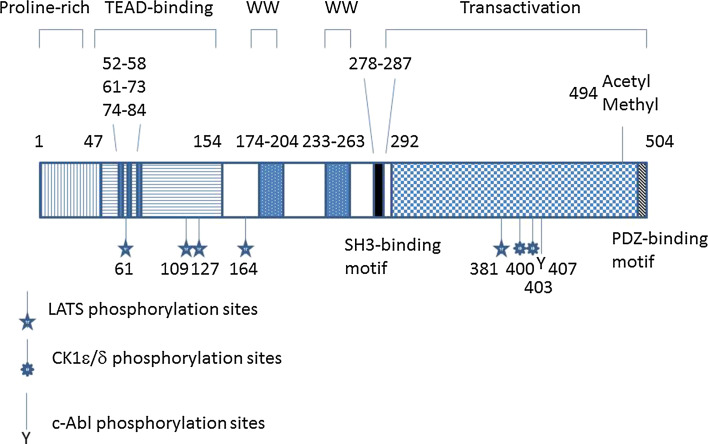
Fig. 1.
Molecular structure of YAP1. In this review, amino acid residues for human YAP1-2γ are used. The TEAD-binding domain has three interfaces for interaction with TEAD (aa 52–58, β-strand; aa 61–73, α-helix; and aa 74–84, Ω-loop). Stars indicate the five LATS-dependent phosphorylation sites. S127 could also be phosphorylated by Akt. S127 phosphorylation generates the 14-3-3-binding site. Residue S381 phosphorylation primes the phosphorylation of S400 and S403 and leads to protein degradation by the SCFβ-TrCP pathway. Residue Y407 phosphorylation by c-Abl promotes p73-dependent pro-apoptotic transcription