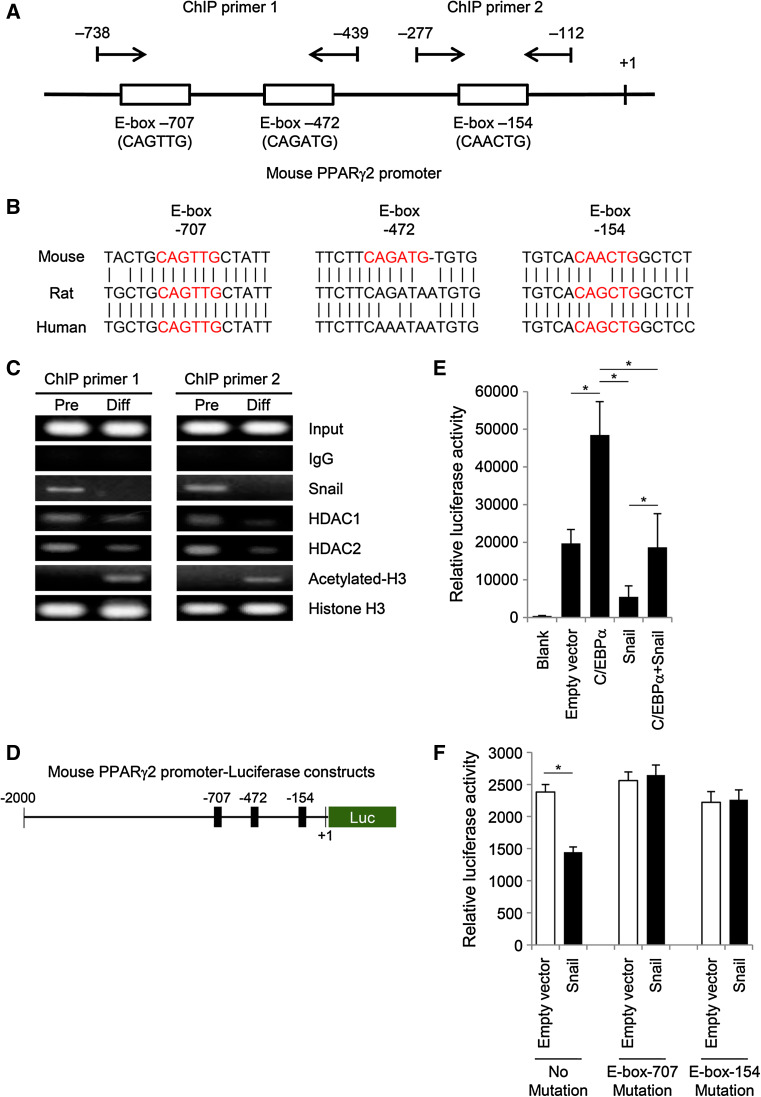
Fig. 2.
Snail inhibits PPARγ expression by directly binding to the E-box in the mouse PPARγ2 gene promoter. a Schematic diagram of the mouse PPARγ2 promoter sequence spanning from –1,021 bp to +1 bp (GenBank: NT_039353.7), with arrows indicating the forward and reverse primers used to amplify ChIP products. Pairs of ChIP primer 1 are located around the two distal E-boxes, which are the possible binding sites for Snail, and one proximal E-box is surrounded by pairs of ChIP primer 2. b E-box motifs in the mouse PPARγ2 gene promoter are conserved in rat and human PPARγ2 gene promoters. Mouse has three putative E-box regions, while rat and human have two E-box motifs (E-box-707 and -154). Predicted E-box motifs in each group are presented in red. c ChIP assay for the upstream region of the PPARγ2 promoter in 3T3-L1 cells was performed using antibodies against Snail, HDAC1, HDAC2, acetyl histone H3, or total histone H3. Presence of the promoter sequence prior to immunoprecipitation was confirmed by PCR (input sample), and immunoglobulin G antibody was used as a negative control. Snail is recruited to the E-box motif of the PPARγ2 promoter in 3T3-L1 preadipocytes, whereas the recruitment of Snail to the PPARγ2 promoter is decreased after differentiation of the preadipocytes into adipocytes. PCR products were separated by electrophoresis on 2 % agarose gels containing ethidium bromide. The results are representative of three independent experiments. d Schematic diagram of the luciferase reporter assay plasmid containing the mouse PPARγ2 promoter. The number indicates the 5′ end of the PPARγ2 promoter gene. e To confirm whether Snail acts as a transcriptional repressor of PPARγ2 expression by binding to the PPARγ2 promoter, plasmids containing Snail or C/EBPα were cotransfected into 3T3-L1 preadipocytes along with the reporter vector containing the PPARγ2 promoter. Reporter assay showed that Snail suppresses C/EBPα-induced transactivation of the PPARγ2 promoter as well as the basal activity of the PPARγ2 promoter. The pGL3-basic vector and the blank well were used as negative controls, whereas C/EBPα-expressing plasmid was used as a positive control. Luciferase activity was normalized using Renilla expression activity. The data are presented as the mean ± SD of four independent experiments. f To assess whether each putative E-box is functional in vitro, sequences of E-boxes in the mouse PPARγ2 promoter were replaced by site-directed mutagenesis. 3T3-L1 preadipocytes were cotransfected with reporter plasmids bearing mutant E-box-707 or -154 and either Snail plasmid or empty pcDNA3 vector (control). Luciferase activity was normalized using Renilla expression activity. The data are presented as the mean ± SD of three independent experiments *p < 0.001