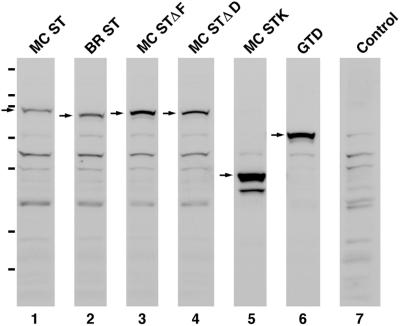
Figure 3.
All six myosin Va tail domain GFP fusions are stable proteins in vivo. Shown are Western blots of whole cell extracts prepared from melan-a melanocytes transfected with each of the six myosin Va tail domain GFP fusion protein constructs described in the text and probed with an antibody to GFP (lanes 1–6). The arrows indicate the positions of the fusion proteins. The remaining bands can be largely if not entirely accounted for by bands appearing in the blot of untransfected melan-a cells (lane 7). The hash marks to the left indicate the migration of the following markers (top to bottom): 200, 116, 97, 66, 55, 36, and 31 kDa. The molecular masses of all six fusion proteins calculated from these blots are within a few percentage points of their estimated molecular masses based on sequence (MC ST, 95 kDa; BR ST, 89 kDa; MC STΔF, 92 kDa; MC STΔD, 92 kDa; MC STK, 52 kDa; and GTD, 72 kDa). Moreover, all six proteins are largely if not entirely intact (only MC STK shows one stable breakdown product of ∼44 kDa). The differences in the intensities of the background bands in lanes 1–6 are due to differences in the amounts of whole cell extract loaded. The differences in the amount of fusion protein per volume of extract were due largely to differences in transfection efficiencies for the different plasmids.