Abstract
The notochord is an embryonic midline structure common to all members of the phylum Chordata, providing both mechanical and signaling cues to the developing embryo. In vertebrates, the notochord arises from the dorsal organizer and it is critical for proper vertebrate development. This evolutionary conserved structure located at the developing midline defines the primitive axis of embryos and represents the structural element essential for locomotion. Besides its primary structural function, the notochord is also a source of developmental signals that patterns surrounding tissues. Among the signals secreted by the notochord, Hedgehog proteins play key roles during embryogenesis. The Hedgehog signaling pathway is a central regulator of embryonic development, controlling the patterning and proliferation of a wide variety of organs. In this review, we summarize the current knowledge on notochord structure and functions, with a particular emphasis on the key developmental events that take place in vertebrates. Moreover, we discuss some genetic studies highlighting the phenotypic consequences of impaired notochord development, which enabled to understand the molecular basis of different human congenital defects and diseases.
Keywords: Notochord, Vacuoles, Peri-notochordal basement membrane, Signaling, Nucleus pulposus, Chordomas
Introduction
The notochord is the most prominent feature of chordates, which originated more than 550 million years ago. Indeed, the phylum Chordata is named after this structure. Many examples in literature describe the evolutionary origin of this distinct chordate character in the history of metazoan evolution, first studied by Alexander Kowalevsky more than 150 years ago [1, 2] (for a review, see [3–5]). A recent study on the polychaete worm Platynereis dumerilii proposed an earlier evolutionary origin in the most recent common ancestor of chordates and annelids [6]. This work challenged the previous view that considered the notochord as an evolutionary innovation of chordates.
In vertebrates, the notochord arises from the dorsal organizer. The organizer appears during gastrulation and has an essential role in the induction of the embryonic axis. In particular, the organizer mediates the dorsal patterning to the mesoderm, regulates the movements of the ectoderm and mesoderm, and finally provides signals necessary for the induction of the neuroectoderm along the antero-posterior axis [7, 8]. In teleosts, the dorsal organizer (named embryonic shield) gives rise to three different germ layers: the floor plate, the notochord and the hypochord [9, 10]. In particular, the notochord precursor, called “chordamesoderm”, begins as a rod of stacked cells that converge at the embryonic midline and express specific markers, such as echidna hedgehog (ehh), sonic hedgehog (shh), collagen II (col2a1), and no tail (ntl) [11]. During the segmentation stage, the expression of these early marker genes is turned off and chordamesoderm cells differentiate into two distinct cell subpopulations, through a Notch-dependent mechanism [12]—an outer sheath layer and an inner vacuolated cell layer. The outer cells secrete a thick extracellular peri-notochordal basement membrane composed of several extracellular matrix proteins, whereas inner cells form large fluid-filled intracellular vacuoles that occupy most of the cell volume (Fig. 1). The notochordal sheath and vacuoles have been described in all analyzed vertebrate species, corroborating the essential role of these components during embryonic development [13, 14]. Indeed, the pressure exerted by notochord vacuoles on the peri-notochordal basement membrane endows this structure with a characteristic stiffness and mechanical strength. In this way, a hydrostatic skeleton is generated during development, driving the elongation of the antero-posterior axis before spine morphogenesis [15, 16]. The biogenesis of vacuolated cells was recently clarified in zebrafish, and it has been suggested that these vacuoles are lysosome-related organelles originating through an H+-ATPase-dependent acidification and Rab32 function [16]. Beyond its structural role, the notochord plays critical functions for developing embryos. In fact, it is also a source of secreted growth factors, such as Sonic hedgehog (Shh), which instruct surrounding tissues to acquire specific differentiated fates.
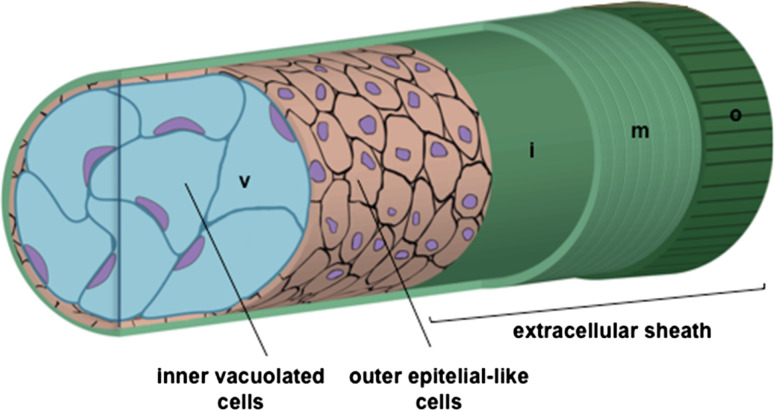
Fig. 1.
Structure of the zebrafish notochord and its surrounding sheaths. This cartoon illustrates notochord structure of inner vacuolated cells surrounded by an epithelial-like sheath of cells. Around these cells, a peri-notochordal BM is present. This extracellular sheath is composed of three parts: the inner (i) basal lamina, surrounded by a medial (m) layer of collagen fibrils that run parallel to the notochord, and an outer (o) granular layer of loosely organized matrix that is mostly oriented in a perpendicular manner to the notochord. The peri-notochordal sheath counteracts the pressure generated from vacuoles (v), providing the rigidity and the correct stiffness of this structure
In this review, we will highlight both the mechanical and signaling role of the notochord, emphasizing the studies performed in zebrafish, one of the major animal models used for the examination of this structure. We will provide an overview of evidences explaining the influence of the notochord on the patterning and maintenance of different neighboring tissues. In the past, elegant mechanistic and genetic studies highlighted that the notochord is a key structural and molecular component of the developing embryo. In more recent studies, some of the molecular mechanisms underlying notochord morphogenesis and function were thoroughly dissected. The full knowledge of notochord structure and function in vertebrates during both early development and late-life stages may enable to better understand different human congenital vertebral defects.
The peri-notochordal basement membrane
Basement membranes (BM) are complex structures composed of different extracellular proteins situated in close contact with cells at various locations in the body. Ultrastructural analysis revealed significant differences in the organization of BMs among different organs [17]. Moreover, there is a large hetereogeneity in the molecular composition of BMs [18, 19], as well as in the mutual interactions and the supramolecular organization of different BM components. The composition of BM together with the repertoire of matrix receptors defines the specificity of cellular responses [20]. The morphology of the peri-notochordal BM is similar among different species, such as teleosts, amphibians and chicken [21]. The peri-notochordal BM is normally composed of three different laminar layers. The single inner layer, in close proximity to the plasma membrane, mainly contains laminins. The intermediate layer is composed of dense collagen fibers oriented in one parallel direction around notochord cells, and distal to this there is an outer layer of fibers oriented in an orthogonal direction [22–24]. This tension-resisting sheath contains vacuolated cells under pressure, resembling the hydrostatic skeleton of different organisms (e.g. worms, herbaceous plants). Biomechanical studies in Xenopus laevis revealed that the role of the notochord sheath in constraining the shape and size of the notochord during development is influenced by the orientation of the sheath fibers [15]. This suggests that the complex fiber architecture of the notochord is related to its peculiar mechanical function.
Work in zebrafish showed that the formation of a notochord sheath is closely linked to the differentiation of notochord cells, and their reciprocal interactions are fundamental for the proper development and function of the notochord itself [12, 22, 25–27]. The fine interplay between cell–cell and cell–matrix interactions during notochord morphogenesis has been elegantly described in X. laevis, uncovering the function of the transmembrane receptor dystroglycan in laminin assembly, cell polarization and vacuole differentiation [28]. The cytoskeletal integrity and the correct stiffness of these cells are essential characteristics that provide resistance to the increasing pressure generated by inflating vacuoles, leading to body axis elongation [28]. In mice, several mutations that specifically affect the development of the notochord have been identified (for a review, see [29]). Mutational studies in zebrafish embryos led to a more comprehensive understanding of notochord development and function. Here we report and discuss previous works describing notochord-related phenotypes due to the loss of different notochord components.
Mutants with early specification defects of the axial mesoderm
The relationships between the dorsal organizer activity and the formation of a mature notochord have been extensively characterized, providing valuable information on the genetic basis of axis formation and notochord development. During early gastrulation, different cellular rearrangements and inductive gene expression patterns promote the transition from the dorsal organizer to chordamesoderm. Analysis of different zebrafish mutant lines revealed two essential loci as key factors for this transition: bozozok (boz) and floating head (flh).
The zebrafish flh gene encodes a homeodomain transcription factor, homologous to Not genes of Xenopus and chick [30–32], which is expressed in the dorsal organizer and notochord during early development [31]. flh mutant embryos entirely lack notochord, which is substituted with muscle tissue in the midline [31, 33]. Thus, flh acts to promote notochord development and repress muscle development in axial mesodermal cells by antagonizing spadetail (spt), a T-box transcription factor normally required for proper morphogenesis and fate specification of trunk somitic muscle cells [34, 35]. Indeed, loss of spt function rescues many aspects of the flh mutant phenotype. spt/flh double mutant embryos develop an anterior notochord, a nearly complete floor plate and do not form ectopic midline muscle, indicating that flh normally antagonizes spt function in the developing midline mesoderm. The data obtained from studies in zebrafish and Xenopus embryos suggest that flh/Not genes are necessary and sufficient to maintain notochordal fate along the entire antero–posterior body axis [31, 36, 37]. Conversely, in mouse embryos, the role of Not appears to be different. Loss of Not function does not affect notochord development in the anterior body axis, but results in abnormal notochord formation in the posterior trunk. This suggests that the role of individual components of the genetic hierarchy governing notochord development vary between different vertebrate species [38].
The zebrafish boz gene encodes for a homeodomain protein named Dharma, previously described for its ability to induce axis formation in the zebrafish embryo [39]. boz mutants display a reduction or a complete loss of the dorsal organizer, with consequent loss of the organizer-derived dorsal midline structures including notochord, prechordal mesendoderm, hypocord and floor plate [40]. Interestingly, the phenotype of boz mutants strongly resembles the one observed after mechanical removal of the embryonic shield, indicating that perturbing in different ways the shield signaling center may cause the same consequences [1]. Moreover, expression of several genes in the embryonic shield is decreased or absent in boz mutants, whereas analysis of endodermal marker, such as axial, showed that only the dorsal mesodermal tissue is affected [40]. At the onset of gastrulation, expression of the notochord-specific flh gene is almost absent in boz embryos [40]. For these reasons, since the embryonic shield does not form in boz mutants and expression of dorsal-specific genes is affected before the shield stage, activity of the Dharma protein is required at the blastula stages for the formation of a complete organizer.
A systematic mutation analysis of early zebrafish development revealed another gene, named momo (mom), which is required for early notochord formation [41]. In mom mutants, the notochord fails to form in the trunk region but appears normal in the tail region. Notably, only one mutated allele of mom has been isolated and its phenotype is not fully penetrant, suggesting that the isolated allele is not a null mutation. mom embryos with a strong phenotype display some similarities with the flh mutants, suggesting that flh and mom have a common role in early specification of the axial mesoderm [41].
Fibronectin is an abundant component of the extracellular matrix throughout the body and it is also found in the peri-notochordal basement membrane of mouse, zebrafish and frog [28, 42, 43]. Despite grossly normal cell fate specification, as well as differentiation and migration of diverse embryonic lineages, fibronectin mutant fish, mouse and frog embryos display severe defects in the overall embryonic morphogenesis and in particular in the formation of somites from somite precursors, differentiation of heart from cardiac precursors, and morphogenesis of the notochordal plate into the notochord [44–49].
Mutants with early notochord defects
In no tail (ntl) and doc (doc) mutants chordamesoderm cells are present in the trunk but then fail to differentiate into a mature notochord. In particular, although ntl mutants develop a BM, the tail does not form and the notochord is completely absent in the most posterior region of the trunk, leading to the fusion of the somites [50]. ntl is orthologous to Brachyury, a well-known gene previously characterized in mouse and Xenopus models [50–53]. ntl encodes for a putative transcription factor that is transiently expressed in both notochord and tail and it is essential for development of both domains [50, 53–58]. Loss of Brachyury/ntl function appears to disrupt morphogenesis of mesoderm during gastrulation in both mouse and zebrafish [59, 60] suggesting that ntl plays an early role in cell fate choice at the dorsal midline to antagonize floor plate development and promoting the notochord formation. Moreover, experiments conducted in ntl/flh double mutants revealed that these two proteins cooperate during chordamesoderm differentiation with a predominant effect of ntl on flh.
In addition to ntl, in doc mutants the chordamesoderm fail to differentiate in most parts of the embryo, despite the formation of a peri-notochordal BM. In doc mutants notochord cells fail to form normal vacuoles and mutant embryos show a reduction of the main axis. The analysis of ntl expression in doc mutants and the similarities of the two mutant phenotypes suggest that doc is required for the maintenance of ntl expression after gastrulation, which in turn is necessary for the differentiation of the chordamesoderm into notochord [41].
Mutants with late notochord defects
Several studies mostly exploited in zebrafish revealed that the BM is not only an essential structural component of the notochord, but it is also required for its right morphogenesis [23]. Although the precise molecular composition of this structure is still partially unknown, during the final stages of notochord development, several proteins have been characterized as essential constituents of the peri-notochordal BM.
Laminins are a family of heterotrimeric extracellular glycoproteins and are the major components of basal lamina of many different tissues. Laminins are composed of a central α-chain, with a varying number of globular regions, and two chains (β- and γ-) with helical and globular regions. The trimeric proteins interconnect cell membranes and extracellular matrix molecules and also bind to each other to form sheets in the basal lamina [61]. The first genetic alterations causing abrogation of notochord sheath formation were identified in the laminin β1 and laminin γ1 genes in the zebrafish [22]. Lamc1 null mouse embryos die too early (E5.5) to study the role of laminin γ1 in notochord sheath formation in this model [62]. In grumpy (gup) and sleepy (sly) zebrafish mutants, in which the zygotic supply of the laminin β1 or γ1 chain is disrupted, laminin 1 protein (or other laminin isoforms containing the β1 or γ1 chain) is absent in the whole embryo, causing at least two types of defects. First, electron microscopy analysis showed a disorganization and loss of the three layers of peri-notochordal BM. This phenotype indicates that laminin 1 is an essential component of this structure and it might serve as a scaffold for the normal organization of other BM components [22]. Moreover, notochord cells fail to form proper vacuoles resulting in a significant shortening of the main embryonic axis, in agreement with the fact that the BM has to sustain the hydrostatic pressure generated from vacuolated cells. Finally, in grumpy (gup) and sleepy (sly) mutants, where laminin β1 or γ1 chains are disrupted, notochord cells do not differentiate, as revealed by the persistent notochordal expression of early expressed genes, such as collagen type II (col2a1), Sonic hedgehog (shh) and Echidna hedgehog (ehh). This phenotype suggests that signals coming from BM proteins are required for notochord differentiation and survival, albeit the molecular mechanisms underlying this process are still unknown. Transplantation experiments provided the link between laminin deposition and notochord differentiation: when gup or sly mutant shields were transplanted to wild-type hosts, a resulting secondary axis was formed with phenotypically normal notochord. Therefore, laminin β1 and laminin γ1 can non-autonomously rescue notochord differentiation. Moreover, wild-type shield cells transplanted into mutant hosts also form normal notochords. Hence, functional laminin chains can be supplied to the notochord from either autonomous or non-autonomous cell sources [22].
The zebrafish locus bashful (bal) encodes for laminin α1 [63]. Mutants lacking this laminin chain display a weaker phenotype compared to gup and sly mutants, since only the anterior region of the notochord fails to form. Interestingly, while laminin 1 immunoreactivity is totally ablated in gup and sly mutants, bal mutant embryos display laminin 1 deposition in the posterior notochord. This data suggests that other laminin isoforms have a redundant role in posterior notochord development. Indeed, knockdown of laminin α4 and α5 chains by injection of antisense morpholino oligonucleotides (MOs) in bal null embryos results in a more severe notochord phenotype, comparable to the one observed in gup and sly mutants [63]. In particular, in laminin α4-MO injected bal mutant embryos vacuoles fail to inflate properly, ehh expression is abnormally persistent and laminin 1 immunoreactivity is severely reduced. Injection of laminin α5-MO in bal mutants leads to the same abnormal and persistent expression of ehh along the notochord, concurrently with failure of cellular inflation and loss of laminin immunoreactivity. Taken together, these results pointed to a redundant role for laminin 411 (also called laminin 8) and laminin 511 (also called laminin 10) in chordamesoderm differentiation and BM assembly [63].
Together with laminins, other extracellular matrix proteins localized in the peri-notochordal BM have an essential role during notochord development. Collagens, the most abundant extracellular matrix proteins, are composed of three polypeptide α-chains coiled around each other to form the triple helical configuration. Depending upon the collagen type, the molecule can be composed of either three identical α-chains (homotrimers), or two or three different α-chains (heterotrimers). Collagens assemble into different types of extracellular matrix structures such as fibrils (e.g., collagen types I–III), networks (e.g., collagen type IV) and microfilaments (e.g., collagen type VI) [64]. Characterization of one recessive lethal mutant, named Gulliver (gul), revealed a new role for the alpha 1 chain of type VIII collagen (col8a1) in notochord morphogenesis [65]. gul mutant embryos display notochord distortion accompanied by a disorganization of collagen fibers only in the medial layer of the notochord sheath. Interestingly, notochord cells form inflated vacuoles and survive, despite the accumulation of protein aggregates in the rough endoplasmic reticulum. It is reasonable to speculate that the undulated shape of the notochord in gul mutants may be due to the defective peri-notochordal BM architecture that is not able to sustain the hydrostatic pressure generated from vacuolated cells. In addition to col8a1, MO-mediated knockdown of the alpha 1 chain of type XV collagen (col15a1) revealed a new role for this extracellular matrix protein as an essential component of the peri-notochordal BM. In col15a1-depleted embryos notochord differentiation is impaired, as suggested by the reduction of the mean size of vacuoles and persistent expression of the two chordamesodermal markers col2a1 and ehh. Moreover, all the three layers of the peri-notochordal BM appear affected and highly disorganized in col15a1-depleted embryos, with a lower number of less dense collagen fibers in the medial and outer layers and a disruption of the inner layer [25]. A key role for type XXVII collagen (col27a1a and col27a1b paralogs) in notochord morphogenesis has been also identified [66]. Both col27a1a and col27a1b show a dynamic expression in the notochord, closely similar to that displayed by col2a1. Single knockdown of col27a1a or double knockdown of both paralogs leads to a notochord curvature at early developmental stages, together with abnormal cellular clumping along the notochord sheath and protein accumulation beside notochord cell membranes. These results suggest that loss of col27a1a and col27a1b leads to a weak peri-notochordal sheath that is unable to withstand the pressure generated by vacuolated cells within the notochord [66].
A similar (but not identical) phenotype has been observed after the concurrent knockdown of loxl1 and loxl5b, two lysyl oxidase-coding genes expressed in the developing notochord [67]. Lysyl oxidases are copper-dependent enzymes that catalyze the crosslinking of elastin and collagens, thus stabilizing the extracellular matrix and maintaining notochord sheath integrity. MO-mediated knockdown of loxl1 or loxl5b does not result in notochord distortion, whereas loxl1/lox5b double morphants recapitulate the kinked notochord observed also with chemically induced copper deficiency [67]. This finding indicates that these two enzymes have overlapping functions during notochord development. Interestingly, inhibition of lysyl oxidases activity by pharmacological treatments or genetic ablation of loxl1 and loxl5b does not affect the peri-notochordal BM deposition and organization, consistent with the concept that lysyl oxidase inhibition alters the final stages of notochord sheath deposition and organization [67]. In addition, the impaired lysyl oxidase activity appears to prevent the differentiation of vacuolated cells, as suggested from the persistent expression of col2a1. One explanation for this sustained col2a1 expression might come from a general loss of notochord strength and stiffness. Hence, it is likely that the BM is required to sense or to transduce a notochord differentiation signal, although a precise mechanistic explanation is still missing. All reported data provide an interesting model for studying the relationships between BM organization, nutritional deficiencies and copper metabolism disorders, in agreement with the essential role of copper for notochord structural organization [68]. For example, copper deficiency results in severe developmental abnormalities. This is most clearly illustrated by Menkes disease (OMIM #309400), a rare X-linked disorder of copper metabolism [69]. Interestingly, as Menkes disease models and to study the effects of developmental copper deprivation, zebrafish calamity (cal) and catastrophe (cto) mutants represent a valuable tool for high-throughput chemical screenings [68, 70].
A forward genetic screen for mutants displaying notochord sensitivity to lysyl oxidase inhibition pointed out a role for the extracellular matrix protein fibrillin-2 in notochord morphogenesis [71]. Fibrillin-2 mutants (puff daddy) and morphant embryos display notochord distortion with a marked reduction of the peri-notochordal BM outer layer size, while the other two layers appear normally organized. Moreover, as observed also in col2a1-depleted embryos [67], fibrillin-2 mutants are sensitized to lysyl oxidases inhibition obtained by loxl5b MO-mediated knockdown or by pharmacological treatment. This sensitivity may be caused in part by an impaired ability to bind and recruit lysyl oxidases at their correct site of action during notochord formation, as already described in knockout mice for other microfibril-associated proteins such as elastin and fibulin-5 [72].
More recently, a similar phenotype has been described for Emilin3, a glycoprotein of the extracellular matrix localized in the peri-notochordal BM at early stages of zebrafish development [27]. The concurrent knockdown of both Emilin3 paralogs (emilin3a and emilin3b) leads to a marked distortion of the notochord associated with a significant shortening of the main embryonic axis. Moreover, fibers of the medial layer of the peri-notochordal BM are disorganized and frequently disrupted in Emilin3 morphant embryos, whereas the outer and inner layers appear normal. Interestingly, quantification of total and vacuolated notochord cells in control and Emilin3 depleted embryos did not reveal any significant difference, suggesting that Emilin3 deficiency is not grossly perturbing the morphology and differentiation of notochord cells [27]. Although the phenotype is not as severe as in laminin mutants, also in Emilin3 double morphants the early chordamesodermal marker col2a1 is persistently expressed, supporting the concept that defects in the notochord sheath can influence the gene expression pattern of notochord cells. However, in contrast to other models of notochord disruption, shh and ehh are overexpressed only in the expression field of emilin3a and emilin3b paralogs, at the level of the chordoneural hinge [27]. Conversely, laminin mutants and collagen XV morphants display a similar upregulation of col2a1 and ehh in the notochord [22, 25]. This implies that notochord cells might respond to extracellular signals in a region-specific manner.
In addition to the genetic mutants and morphant models showing defects in later notochord formation, three other mutants have been characterized for their defects during the formation of a mature notochord. Sneezy (sny), happy (hap) and dopey (dop) loci encode the α, β and β′ subunits of the zebrafish coatomer complex [73]. This complex consists of seven subunits that in association with the small GTPase Arf1 form the vesicular coat of COPI vesicles. Coatomer complex is essential for the maintenance of the Golgi apparatus and the control of retrograde vesicular transport machinery. Hence, in the absence of COPI function, the normal transport of proteins from endoplasmic reticulum and Golgi is disrupted (for a review, see [74]). During chordamesoderm differentiation there is an elevated secretory activity and a higher demand for coatomer function in the notochord. For this reason, the mRNA expression is specifically upregulated during the transition from the chordamesoderm to the notochord [73]. Loss of COPI function in sny, hap and dop mutants produce almost identical morphological defects, including a shortening of the main axis due to the loss of notochord cell differentiation. This defect is confirmed by the persistent expression of the chordamesoderm markers shh and col2a1 and the generation of vacuoles that fail to fully inflate and undergo to apoptosis [73]. Moreover, electron microscopy showed that, while the innermost layer of the peri-notochordal BM is correctly deposited and display normal levels of laminin 1 immunoreactivity, the medial layer is mostly disrupted [73]. Shield transplantation experiments showed that the medial layer of the peri-notochordal BM derives only from the chordamesoderm and, in contrast to the inner layer, cannot be supplied by exogenous sources [73]. These findings indicate that the notochord has a strict requirement for coatomer function in order to undergo its correct development and organization.
A number of other mutations were isolated in zebrafish, leading to a variety of other late notochord defects. For example, in crash test dummy (ctd), zickzack (ziz), quasimodo (qam), kinks (kik) and wavy tail (wat) mutants, the common phenotype is an undulated notochord along the body axis [41, 75].
Taken together, these studies in zebrafish mutant and morphant models highlighted the importance of the correct deposition and organization of the peri-notochordal BM during notochord development. First, the peri-notochordal BM is important for the elongation of the embryonic axis, conferring the proper rigidity to the developing embryo. Alterations of this structure may prevent the mechanical support needed to withstand the hydrostatic pressure originated from vacuoles. Although the various studies discussed here support the concept that the peri-notochordal BM is necessary for notochord differentiation, it remains unclear what is the relationship between the inability to form an extracellular sheath and the failure to differentiate. It is reasonable to speculate that loss of the BM may lead to loss of BM-associated signals, including gene expression, apoptosis, proliferation and regulation of growth factors. The analysis of other notochord mutants will be useful to obtain additional insights into the mechanism of signaling related to the BM and the notochord. The ability to knockdown candidate genes of interest using MOs had an important role in the application of the zebrafish as a model system. However, MOs can induce p53-dependent apoptosis [76–78], and several off-target phenotypes were described in a recent systematic phenotypic analysis of a large number of morphant embryos [79]. These shortcomings strongly raise the concept of reconsidering the use of antisense-based technology for characterization of gene function. The application of programmable site-specific nucleases or the recently developed CRISPR/Cas9 technology for in vivo targeted gene disruption may provide a much better option to characterize gene function during development.
Notochord signaling
In addition to its structural function, the notochord is also an important source of signals that are essential for patterning the adjacent tissues [23, 80]. In the sections below, we will discuss the experimental evidence indicating that the notochord is a source of key signaling molecules for the surrounding ectodermal, mesodermal and endodermal derived tissues (Fig. 2).
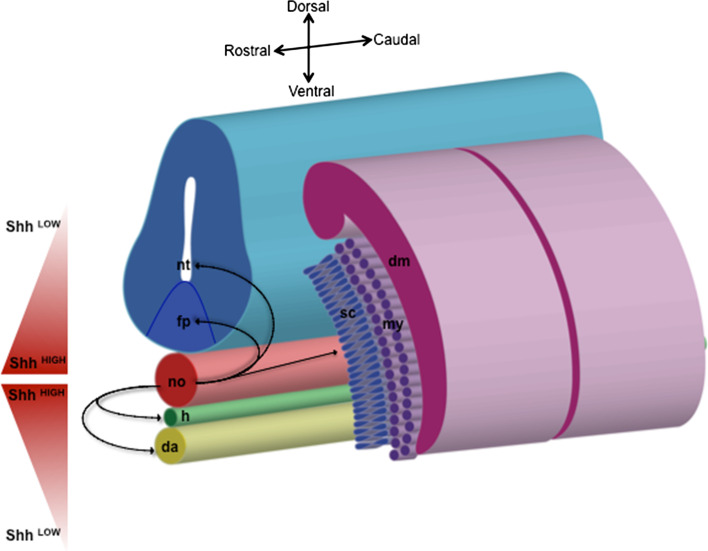
Fig. 2.
Schematic diagram of transverse sections of a vertebrate embryo depicting notochord signaling to adjacent tissues. The notochord (no) secretes Shh, represented by arrows in the figure. This signal is responsible for patterning the ventral neural tube (nt). In particular, the notochord is able to induce the differentiation of the floor plate (fp), and they both represent a continuing source of ventralizing signals within the neural tube. The notochord is also important for somite differentiation, where Shh concentration gradients differentially pattern dorsal–ventral somitic fates. Very low levels of Shh signal to maintain the dermomyotome (dm); slightly higher levels induce both loss of dermomyotomal markers and activation of myogenic differentiation (myotome, my); finally, higher levels of Shh cause the loss of myotomal markers and the induction of sclerotome (sc). Notochord signaling is also critical for the development of the hypochord (h) and of the dorsal aorta (da)
Notochord signals for ectoderm development
The ectoderm is divided into three major domains: the outer ectoderm (mainly the epidermis), the neural tube (brain and spinal cord), and the neural crest (peripheral neurons, pigment, facial cartilage). During vertebrate embryonic development, signals from the dorsal organizer or its derivative, the notochord, are responsible for ectodermal commitment to a neuronal lineage.
Patterning the neural tube
Patterning of the neural tube is perhaps the best characterized role for the notochord during embryonic development. The vertebrate nervous system arises from the neural plate, a sheet of neuroepithelial cells that overlies the axial mesoderm. As development proceeds, the neural plate folds to form the neural tube, which in turn will form the spinal cord and the brain. At this stage, a dorsoventral polarity is established: cells in the dorsal half generate different neurons that relay sensory signals, whereas cells in the ventral half generate distinct classes of motor neurons and ventral interneurons that participate in the coordination of motor output (for a review, see [81]). Pioneering observations and embryological manipulations in chick and amphibians indicated that the notochord has a key role in establishing the dorsoventral polarity of the spinal cord [82, 83]. The notochord-secreted protein Shh was found to be the signal required and sufficient for patterning the ventral neural tube. The importance of Shh is confirmed by the loss of ventral neurons in embryos in which Shh signaling was either biochemically or genetically blocked [84–88]. Shh is initially secreted by the notochord and is able to induce the differentiation of the floor plate, a specialized group of neuroepithelial cells in the ventral portion of spinal cord [83, 89–91]. Then, floor plate cells express themselves shh, conferring the capability to specify both floor plate and motor neuron cellular fates, thus establishing a continuing source of a ventralizing signal within the neural tube. Evidences indicate that the minimal amount of Shh sufficient to induce the differentiation of each neuronal class in vitro correlates with the distance of Shh from its source of synthesis in vivo [88]. Thus, neurons generated in progressively more ventral regions of the neural tube require higher Shh concentrations for their induction [88]. Moreover, the generation of an engineered mouse that produces fluorescently labelled Shh from the endogenous locus provided new insights into the possible mechanism of in vivo Shh signal distribution during neural tube patterning [92]. This study supports a model wherein a graded signaling response to notochord-derived Shh activates a long-range patterning process in the ventral neural tube. Indeed, when patterning begins, Shh ligand is first detected at the apical region of ventral midline cells and then is progressively extended to the dorsal region. Moreover, the temporal gradient of Shh ligand accumulation is similar to the in vitro profile of Shh concentrations that is needed to progressively specify more-ventral cell types [92].
Although the analysis of floor plate differentiation in avian and mammalian embryos provided a coherent picture of the inductive process, genetic studies in zebrafish revealed that a differentiated notochord is not strictly required for the generation of a floor plate in this vertebrate. Indeed, zebrafish mutants lacking the notochord (flh, momo, bozozok, and ntl) and shh mutants can develop al least the medial floor plate [93, 94]. In addition to shh, zebrafish embryos express two other Hh-related genes, echidna hedgehog (ehh) and tiggywinkle hedgehog (twhh), in notochord and floor plate respectively. Therefore, it is reasonable that multiple Hh genes might cooperate in the induction of a floor plate in zebrafish. Conversely, it is likely that only shh is responsible for the floor plate differentiation in avian and mammalian embryos. In fact, in chick and mouse embryos the specification of the floor plate and other ventral neuronal progenitors depends on distinct timing and duration of Shh signaling [95]. In a restricted developmental time frame, spanning from gastrulation to early somitogenesis stages, a transient burst of Shh signaling from the notochord is both necessary and sufficient for the specification of floor plate [82, 84, 87]. However, neural progenitors progressively lose their ability to acquire a floor plate identity in response to high levels of Shh. The downregulation of Shh signaling is a prerequisite for the onset of a floor plate identity [95]. Furthermore, it is likely that this mechanism contributes to the restriction of the floor plate to the neural tube midline. The possibility that neuroepithelial cells can generate a floor plate in response to other signaling pathways active within neural tissue cannot be excluded [95]. Notably, concomitant to Shh, signals of the bone morphogenetic protein (BMPs) family coming from the epidermal ectoderm control dorsal neural tube cell fate and modify its response to Shh signaling, by inhibiting the specification of a ventral cell fate [96–98]. In this context, the presence of notochord-derived BMP antagonists (e.g. Noggin, a notochord-secreted signaling molecule that binds and inactivates BMP, therefore playing a role in generating morphogenic gradients) allows to generate a permissive environment for Shh-mediated induction of the floor plate [99]. Notably, together with BMP ligands, other factors may also influence neural cell responsivity to Shh signaling. Interestingly, studies in chick embryos demonstrated that vitronectin, an extracellular matrix protein expressed in the notochord, is able to potentiate the motor neuron inducing activity of Shh through direct binding to Shh ligands [100, 101].
Patterning other ectoderm derivatives
Different literature data suggest that the notochord, together with the ventral diencephalon and the mesenchyme, is involved in the development of the anterior pituitary. During the formation of cephalic tissues, the rostral tip of the notochord is adjacent to the cephalic ectoderm, where the anterior pituitary starts to develop. Indeed, transplantation experiments revealed that the anterior notochord is able induce the outpouching of the head ectoderm [102]. This leads to a partial formation of the Rathke’s pouch and the upward invagination of the oral ectoderm, giving rise to the anterior pituitary. Therefore, a close contact between the diencephalon and ectoderm is a prerequisite for the induction of the anterior pituitary [102].
Notochord signals for mesoderm development
The murine notochord, which is present from embryonic day (E) 7.5 until E12.5, forms all cell types of the nucleus pulposus in intervertebral discs (IVDs) [103, 104]. Moreover, temporal removal of Shh ligands from both the notochord and floor plate demonstrated that this signaling pathway is required for the formation of the peri-notochordal sheath and subsequently of the vertebral column [104]. More recently, it has been found that the expression of Shh in the notochord is sufficient to pattern the intervertebral discs and vertebral column. Indeed, removal of Shh from the notochord leads to failed Shh expression in the floor plate and results in the loss of vertebral structures and nuclei pulposi. In contrast, removal of Shh from the floor plate results in phenotypically normal vertebral column and intervertebral discs. These data indicate that the expression of Shh from the notochord is sufficient for patterning the entire vertebral column [105].
Patterning the somites
During vertebrate embryogenesis, the paraxial mesoderm that lies adjacent to the neural tube becomes segmented into paired epithelial somites. Then, each somite differentiates into the ventral sclerotome, from which the skeletal elements of the ribs and vertebral column derive, and the dorsal dermomyotome. The dermomyotome further differentiates into the dorsal-lateral dermotome, giving rise to dermis and myotome. The myotome is localized between the sclerotome and the dermotome and represents the precursor of axial musculature. Multiple extracellular signals expressed in the surrounding tissues determine the dorsal and ventral somitic cell fates, establishing the number of precursors that will give rise to either cartilage, muscle or dermal cells (for a review, see [106]).
It has been shown that the initiation and maintenance of the sclerotome depends on both Shh and Noggin, a Bmp4 antagonist, which are both expressed in the notochord when the sclerotome formation begins. Shh produced by the notochord and the floor plate is also important for the proliferation and survival of sclerotomal cells [87, 107–111]. Indeed, in mice with ablation of Hh signaling, sclerotome formation is compromised and no vertebral column is formed [84, 112]. Conversely, gain-of-function studies have demonstrated that ectopic expression of Shh induces the expression of the sclerotomal marker Pax1 and inhibits the expression of the dermomyotomal marker Pax3 [108, 113]. Moreover, it has been demonstrated that the activity of notochord-derived Noggin is required for sclerotome differentiation, since Noggin-deficient embryos display defective sclerotome formation [114]. Indeed, Noggin inhibits the repressive activity of BMP signals, thus allowing the Shh-mediated induction of Pax1 in the somitic mesenchyme and the sclerotomal cell growth and differentiation into cartilage [114]. Therefore, it can be assumed that both signals, Shh and Noggin, cooperate during sclerotome formation [115]. In spite of this, studies in the Danforth’s short-tail (Sd) mutant mouse, carrying a semi-dominant mutation that affect vertebral column development, challenged the concept that notochord might be required for sclerotome formation. In the Sd mouse model, although the notochord degenerates completely by E9.5, the floor plate is sufficient to maintain the differentiation of somites into sclerotomes and vertebrae [116]. More recently, it has been found that notochord-derived Shh regulates the expression of the chick early B cell factor 1 (cEbf1), a medial sclerotome marker expressed in somite areas close to the notochord that plays a crucial role for sclerotome development and formation of the vertebral body and pedicles [117].
In vitro and in vivo studies demonstrated that notochord-derived Shh promotes the myotome cell fate [118–121]. Mouse embryos lacking Shh or its cell membrane ligand Smoothened display markedly decreased expression of Myf5, a key myogenic regulatory factor [84, 112]. In zebrafish, Shh is required for the differentiation of Pax3/Pax7-expressing dermomyotomal cells into MyoD-positive differentiated myocytes. In the absence of Shh signaling, Pax3/Pax7-expressing cells increase in number, but fail to activate the myogenic program [122, 123]. Even in this context, the Shh concentration gradients work in a morphogen-like fashion to differentially pattern the dorsal–ventral somitic fates [124]. Indeed, different Shh levels elicit distinct responses in somitic cells. Very low Shh levels, combined with Wnt signaling activity, elicit the maintenance of dermomyotomal gene expression (Pax3). Slightly higher Shh levels induce both loss of dermomyotomal marker gene expression (Pax3) and activation of myogenic differentiation (Myf5 and MyoD). Finally, high Shh levels cause the loss of myotomal markers and the activation of sclerotomal gene expression [124].
It is known that dermomyotome progenitors colonizing the myotome first acquire a myocyte identity and subsequently proliferate as Pax7-expressing progenitors, before undergoing terminal differentiation. Recently, it was shown in both avian and mouse embryos that the loss of Shh responsiveness in dermomyotome-derived muscle progenitors is a key mechanism by which the myotome transits from differentiation into expansion [125]. Initially, Shh emanating from the notochord/floor plate through the sclerotome is able to reach the dermomyotome. In the dermomytome Shh both maintains the proliferation of dermomyotome cells and promotes the terminal myogenic differentiation of progenitors colonizing the myotome. Subsequently, when the myotome enters in the growth phase, myogenic progenitors become refractory to differentiation stimuli coming from Shh [125].
Studies in zebrafish revealed that the correct specification of different cell types within the myotome is also dependent on the timing at which they receive the signal from the notochord and on their competence to respond [126]. Initially, the exposure of myotomal precursors to Hh is restricted to adaxial cells, a single cell layer lying close to the embryonic shield. Then, notochord-derived Hh proteins induce adaxial cells to become slow-twitch muscle fibers [127]. The early exposure to Hh ligands seems to be essential for the specification of muscle pioneer precursors, the slow muscle fiber myoblasts that respond to the highest levels of signaling and express engrailed genes [128]. A minor component of muscle pioneers become fated to form the superficial slow fibers and move away from the notochord, displacing precursors of the fast twitch fibers. By this time, these cells—called medial fast fibers—are irreversibly committed to the fast lineage; nevertheless they retain the ability to respond to Hh, as evidenced by their activation of engrailed expression, though at lower levels than muscle pioneers [127].
Many zebrafish mutants affecting notochord development, such as flh, mom, ntl and doc, display a loss of muscle pioneers and of the horizontal myoseptum, the structure that separates somites into a dorsal and a ventral part. Indeed, cell transplantation experiments carried out in ntl and doc mutants showed that somite defects can be rescued by exogenous wild-type notochord cells [31, 41, 50, 129]. Moreover, flh mutant embryos display the transdifferentiation of notochord to muscle [33], and an ectopic induction of both myf5 and myoD in midline cells, especially in caudal somites. Indeed, abolishment of notochord-derived signals in flh mutants leads to extensive slow myogenesis, indicating that the combined action of floor plate-derived Twhh and notochord-derived Shh are sufficient to support non-pioneer slow myogenesis [33]. However, Twhh and Shh are unable to maintain and reinitiate MRF expression in adaxial cells during late stages of development. In fact, complete loss of ventral midline signals results in the absence of caudal slow muscle fibers and the formation of fast muscle fibers. Taken together, these data suggest that Shh released from the notochord is required for the maintenance of MRF expression. The additional exposure to midline signals reinitiates MRF expression and delays slow muscle fibers formation in flh and ntl mutants [130].
Patterning other mesodermal tissues
Notochord signaling is also important for the development of heart and vasculature. Previous studies in zebrafish showed that the anterior end of the notochord normally suppresses cardiogenic fate, by delimiting the expression of Nkx2.5, the earliest marker of presumptive myocardial cell fate [131]. Furthermore, the notochord is involved in the specification of the cardiac left–right asymmetry. Actually, when the notochord is surgically ablated or when it is absent in mutant embryos (such as ntl and flh zebrafish mutants and no turning mice mutants), asymmetric markers of lateral plate mesoderm are either randomized or bilaterally expressed [132–134]. Recently, it has been demonstrated that Hh signaling is required for the earliest steps of specification, differentiation and proliferation of endocardial progenitors in the zebrafish embryo. The Hh signaling is cell-autonomously activated within endocardial progenitors, and the notochord is likely a source of this Hh signal [135].
Concerning the vasculature specification, the notochord seems to have distinct roles in different species and developmental stages. The endothelial precursor cells or angioblasts originate from the mesoderm and then disperse throughout all embryonic tissues [136, 137]. The axial vessels of the trunk form in close proximity to the notochord and the endoderm. The dorsal aorta is normally just ventral to the notochord, whereas the axial vein is just below the dorsal aorta and above the endoderm. Studies in zebrafish indicate that the differentiated notochord is strongly required for the development of the dorsal aorta [138]. In fact, a fully developed notochord is needed for the expression of the vascular endothelial growth factor receptor (VEGFR2) in this axial artery and for the normal vessel assembly [138]. Conversely, the notochord seems less important for the development of the axial vein [139]. Indeed, in both flh and ntl mutants the dorsal aorta fails to form, while formation of the axial vein is less affected [138, 139]. The less severe vascular phenotype observed in ntl mutants may be caused by residual notochord signaling or alternatively by floor plate signals, which share many inductive properties with the notochord and could compensate for notochord loss [138, 139]. Experimental analysis showed that during somatogenesis notochord-derived Hh signals regulate the expression of vegfa, coding for vascular endothelial growth factor A (VEGF-A) in the somitic mesoderm. In turn, VEGF-A acts on endothelial cells to drive arterial differentiation [140]. During early avian development, blood vessels develop in the embryonic disc, except in a midline region surrounding the notochord. Indeed, notochord-derived BMP antagonists, including noggin and chordin, are responsible for the generation and maintenance of this midline avascular region by preventing endothelial cells differentiation, migration, and network formation [141]. The combination of these inhibitory cues ensures that blood vessels are excluded from the midline until the paired dorsal aorta fuse.
A narrow strip of tissue located between the paraxial and lateral plate mesoderm, called the intermediate mesoderm, gives raise to the vertebrate kidney. During embryogenesis, three sets of structures (pronephros, mesonephros and metanephros) emerge from the nephric cord within the intermediate mesoderm in an antero-posterior direction. It has been demonstrated that the notochord and floor plate-derived Shh ligands are dispensable for nephrogenesis but required for the correct positioning of the bilateral kidney primordia [142]. Actually, ablation of the notochord and floor plate in mice displays no effect on nephrogenesis but leads to kidney fusion [142]. A recent study on the temporal analysis of Shh function—using a tamoxifen-inducible gene recombination system—indicated that midline-derived Shh is essential to regulate the number of mesonephric tubules [143].
Notochord signals for endoderm development
The endoderm germ layer gives rise to the epithelium of the pharynx and of the respiratory and gastrointestinal tracts, including thyroid, thymus, lung, esophagus, stomach, intestine, liver and pancreas. Thus, determining how the endoderm is patterned is crucial for understanding the development of many internal organs.
The pancreas forms from the fusion of distinct dorsal and ventral buds. Embryonic manipulation in chick provided strong evidence that early signals released from the notochord to the endoderm permit dorsal pancreatic morphogenesis [144]. Moreover, these signals initiate and maintain the expression of different genes required for pancreatic development. In contrast ventral pancreas, which does not directly contact the notochord, develops normally in the absence of a notochord. In vitro recombination experiments suggest that notochord provides a permissive, rather that instructive, signal to the endoderm for the specification of a pancreatic fate. Indeed, pancreatic gene expression can be initiated and maintained only in prepancreatic chick endoderm cultured with notochord cells, whereas non-pancreatic endoderm does not express pancreatic genes when recombined with the same notochord [144]. Moreover, grafting experiments demonstrated that activin-βB and fibroblast growth factor 2 (FGF2) expressed by the notochord repress endodermal Shh, thereby permitting pancreatic development [145]. Indeed, notochord removal before the onset of the notochord/endoderm molecular interactions leads to ectopic Shh expression in the pancreatic anlage, abnormal pancreatic morphogenesis, and prevents gene expression required for endocrine and exocrine compartments differentiation. Conversely, the inhibition of Shh in isolated prepancreatic endoderm is sufficient to induce specific pancreatic gene expression [145].
Embryological manipulations in amphibians demonstrated that the notochord plays a critical role in the development of the hypochord, a transient, endoderm-derived structure that lies immediately ventral to the notochord in the amphibian and fish embryo. During early neural stages, signals arising from the notochord instruct cells in the underlying endoderm to reach a hypochord fate, whereas further notochord signals are dispensable for the maintenance of this structure [146]. For example, in flh and ntl zebrafish mutants, the hypochord does not develop or it is severely disrupted [33]. However, in zebrafish gup, bal, dop and sny mutants, which are defective in late notochord development, the hypochord appears normal as assayed by the expression of the co2a1 marker [129]. Removal of the notochord during early neurulation leads to a complete failure of hypochord development and to the loss of vegf expression. Grafting experiments demonstrate that higher levels of notochord-derived signaling can expand the number of vegf-expressing cells, increasing the size of the differentiated hypochord [146].
Recently, using spatio-temporal manipulation of Noggin conditional alleles during foregut development, it has been demonstrated that during early notochord development the action of Noggin results in BMP signaling attenuation, which in turn is required for the correct separation of the notochord cells from the dorsal foregut endoderm [147]. Importantly, this event appears to be a prerequisite for foregut compartmentalization. Indeed, mice lacking the BMP antagonist Noggin have esophageal atresia with tracheoesophageal fistula, as well as defects in lung branching [148]. This phenotype appears to correlate with abnormal morphogenesis of the notochord. In particular, defective separation of the notochord from the definitive endoderm leads to hypocellularization of the foregut just before the critical period of compartmentalization [147].
Role of extracellular matrix proteins in notochord patterning activity
In order to activate different embryonic differentiation programs, the Hh signals arising from the developing notochord must pass through the extracellular matrix surrounding notochord cells. Laminins are found in the peri-notochordal BM around forming somites, and later on around myotomes including vertical myosepta [22]. The source of notochord-derived Shh has an essential role in controlling laminin α1 gene expression. As laminin 111 (also called laminin-1) is necessary and sufficient to initiate BM formation, these studies revealed that Shh plays a key role in this process [149]. In other systems (e.g. in vitro embryonic stem cells) it is known that Smad4, a BMP mediator, controls BM deposition through a dual control of metalloproteinase and laminin α1 synthesis [150]. In zebrafish sly mutants, which carry a mutation in the gene encoding for laminin γ1 thus preventing the formation of laminin 111, Hh signaling is not affected, as revealed by normal expression levels for ptc, a gene that is transcriptionally regulated by this signaling pathway [151]. Indeed, these mutants display ectopic BMP activity in the myotome [152]. These findings suggest that a complex feedback mechanism between Shh, BMP and laminin 111 is responsible for the control of BM deposition and myogenesis.
Another example of mutual interaction between peri-notochordal BM deposition and notochord-related signaling activity has been revealed by zebrafish collagen XV depleted embryos [25]. Morpholino-mediated knockdown of col15a1 revealed that this peri-notochordal sheath protein is required for notochord differentiation, axon guidance and differentiation of functional muscle fibers. The authors proposed that the loss of BM integrity due to ablation of collagen XV may lead to an increased diffusion of Hh ligands from the notochord, resulting in an ectopic differentiation of medial fast fibers. The distinctive expansion of medial fast fibers in this model correlates with the onset of collagen XV expression after the Shh-mediated induction of adaxial and muscle pioneer cells [25].
Studies in puff daddy (pfd), a zebrafish mutant yielded by a forward genetic screen for mutants exhibiting notochord sensitivity to lysyl oxidase inhibition, demonstrated that fibrillin-2 has an essential role in zebrafish notochord and vascular morphogenesis [71]. Although the notochord distortion observed in pfd mutants results from structural defects, the vascular phenotypes appear to rely at least in part upon altered cell signaling pathways. As genetic interaction between fibrillin-2 and bone morphogenetic protein-7 (BMP-7) has been demonstrated in fibrillin-2 knockout mice [153], Bmp-7-related signals or other morphogens may be required for endothelial cell branching and venous plexus morphogenesis in zebrafish. Moreover, treating zebrafish pfd mutant embryos with losartan, a specific inhibitor of the transforming growth factor (TGFβ) signaling pathway, does not rescue their vascular phenotype [154], thus indicating that TGFβ signaling is not involved in the caudal vein abnormalities observed in fibrillin-2 depleted embryos [71].
Recent in vitro and in vivo studies indicate that the peri-notochordal sheath protein Emilin3 modulates the availability of Hh ligands by interacting with the permissive factor Scube2 in the notochord sheath [27]. Scube2 acts non-cell autonomously in the release of lipidated Shh from producing cells [155, 156]. Intriguingly, Emilin3 was shown to interact with the EGF repeats of Scube2, supporting the hypothesis that this extracellular matrix protein may be important for targeting Scube2 to the correct sites, thus tuning its activity in the extracellular milieu. Emilin3 deficiency disturbs notochord sheath formation and may cause an abnormal localization and/or activity of Scube2, leading to increased Hh signals. In agreement with this, expression of ptc1 and other Hh-responsive genes was shown to be consistently upregulated in Emilin3 depleted zebrafish embryos [27].
Role of the notochord in intervertebral disc formation and in spine morphogenesis
During late embryonic development, the peri-notochordal BM exhibits a metameric pattern of more and less condensed zones that give rise to the outer and inner annulus and the vertebrae [157]. While the notochord completely regresses in the correspondence of the vertebral bodies or centra, small areas of notochord tissue persist in the center of the intervertebral discs and notochord cells condense to form the nucleus pulposus [158, 159]. Thus, in chordate embryos, the nucleus pulposus is the only tissue that is completely derived from the notochord [103]. In zebrafish, vertebrae and IVDs become distinct from each other by 15 days post fertilization. The IVDs contain vacuolated cells of the nucleus pulposus surrounded by smaller cells of the anulus fibrosus [160].
Several gene products regulate intervertebral disc development. Mutant mice lacking collagen type II, which is synthesized by the notochord and deposited into the peri-notochordal sheath, lack intervertebral discs and are unable to remove the notochord. This defect is associated with an enlargement of vertebral bodies and the deposition of abnormal collagen fibrils [159]. Two highly related transcription factors, Sox5 and Sox6, which are expressed in sclerotome-derived cells and in the notochord [161] are required for peri-notochordal sheath formation, cell survival, and late development of the nucleus pulposus. Indeed, the peri-notochordal BM fails to form in Sox5/Sox6 double mutant mice, leading to the subsequent impairment of axial skeleton formation [162]. These findings suggest that a mechanically and structurally intact peri-notochordal sheath is required for inducing notochord reorganization, formation of the intervertebral disc and dismantling of the notochord. Two other paired box transcription factors expressed in the sclerotome, Pax1 and Pax9, regulate vertebral body development. In the corresponding mutant mice, the notochord forms normally but it is not removed from the vertebral bodies, thereby compromising the formation of the nucleus pulposus [163, 164].
The vertebrate notochord plays a critical role in spine morphogenesis and bone ossification during late developmental stages. The link between notochord cells and spine morphogenesis has been deeply analyzed in the Atlantic salmon [165], but in the last years the zebrafish model has become a useful tool to understand the mechanisms underlying the formation of bone matrix of the vertebrae. In teleosts, the notochord directs centrum bone synthesis in vivo, where individual rings of bone centra are deposited within the notochord sheath, leading to the subsequent formation of mature segmented vertebrae [165, 166]. An in vivo study focused on the development of the zebrafish vertebral column revealed that laser ablation of notochord cells at defined periodic locations prevents the development of the correspondent centrum [167]. These findings suggest that the notochord might have an inherent periodicity that imparts segmental patterning to the vertebral column. Moreover, it has been demonstrated that the fully inflated vacuoles of notochord cells mechanically antagonize the pushing force of invading osteoblasts during vertebral bone formation. Zebrafish mutants lacking fully vacuolated cells or with fragmented vacuoles developed kinks in the spine during ossification [16]. These findings identify a novel role for notochord vacuoles in spine morphogenesis. Different works support the model of notochord-dependent vertebral column formation [16, 66, 167, 168]. Thus, it is reasonable to suppose that the patterns of vertebral alterations and scoliosis can be a common consequence of malformations or injury to embryonic notochord tissues. Further studies will help elucidating the mechanisms underlying vertebral column ossification.
As the intervertebral disc matures, the composition of the nucleus pulposus changes: the large vacuolated cells with notochordal origin decrease in number, whereas smaller chondrocyte-like cells, of a different embryonic origin, increase. Indeed, there is a controversy regarding the embryonic origin of the cells populating the mature nuclei pulposi. Some authors’ stated that notochord cells die during spine morphogenesis and then are replaced by a new population of cells migrating from adjacent tissues [169]. Other authors, however, argued that the smaller adult nucleus pulposus cells, despite their distinct morphology, derive from the original population of larger notochord cells [105]. Some recent reports unveiled the possibility that a notochord-derived population of adult nucleus pulposus cells coexists with another cell population having a different ontogeny (for extensive review on this topic see [170]).
Role of the notochord in the onset of chordomas
A number of animal models of intervertebral disc degeneration were generated [171–176]. However, in these models the fate of the notochord cells, as well as their involvement in functional maintenance of the intervertebral disc in postnatal life, remains poorly defined. For example, few dormant notochord cells persist in the adult vertebral body and give rise to chordomas [103]. Chordomas are rare primary tumors of the axial skeleton, localized mainly in the sacrococcygeal and sphenooccipital regions [177]. These tumors are both sporadic and hereditary and appear to occur more frequently after the fourth decade of life. However, modern technologies have increased the detection of pediatric chordomas. They are traditionally considered to be slow growing, locally invasive neoplasms with a low tendency to metastasize and a high potential for recurrence. Complete surgical removal of the tumor, whenever possible, is the standard of care, usually in combination with radiation therapy. In fact this tumor is known to be highly chemoresistant towards the few proven systemic therapies available for patients with unresectable tumors or distant metastases [178].
The current concept is that chordomas arise from the remnants of the notochord after embryogenesis. Lineage-tracing experiments in mouse models allowed to clarify that during embryogenesis, as the ossification of the spine proceeds and vertebral bodies form, the embryonic notochord directly gives rise to all cell types present in the nucleus pulposus of the intervertebral discs. Additionally, rare isolated embryonic notochord cells were also found to reside in the vertebrae between the intervertebral discs. The location in which these cells are found is characteristic of the “notochordal remnants”, which in humans have been proposed to give rise to chordoma [103]. Other observations support the notochordal origin of chordomas. Between notochordal remnants and the histopathology exhibited by chordomas there is a morphological similarity at light and electron microscope levels [179, 180], and both share a similar immunophenotype [177, 181, 182]. Histologically, chordomas are composed of physaliphorous cells expressing a particular low-molecular-weight cytokeratin protein embedded in a mucomyxoid background. They are divided into classical (or conventional), chondroid, and dedifferentiated types. The chondroid variety may have a more favorable prognosis than other chordomas [183].
Actually, chordomas do not only have microscopic features resembling the embryonic notochord, but also express the transcription factor T (referred to as brachyury henceforth), the founding member of the T-box family of transcription factors, which is required for the specification of mesodermal identity [184] and regulates notochord formation. Duplication of the brachyury locus was reported in rare cases with genetic predisposition to familial chordoma [185], along with amplification of brachyury in some sporadic chordomas [186]. Moreover, an extensive genotyping study of individuals with sporadic chordoma revealed recurrent single nucleotide polymorphisms (SNPs) in the brachyury DNA-binding domain. These SNPs alter the DNA-binding properties of the transcription factor, suggesting that misregulation of some of the genes controlled by brachyury could be a mechanism underlying the genesis of chordoma [187]. A recent study using integrated functional genomics identified several target genes of brachyury involved in the regulation of the cell cycle, in the production of extracellular matrix and cytokines, and in glycosaminoglycan binding, all of which are pertinent to the notochord and chordomas [188]. At a functional level, in vitro suppression of brachyury halts cell proliferation in two different chordoma cell lines [186, 189]. It was also shown that overexpression of brachyury leads to enhanced proliferation, motility, and invasiveness in a brachyury-depleted cell line [190]. Altogether, these findings implicate brachyury in the pathogenesis of chordoma and suggest that this transcription factor is a crucial molecular driver in the initiation and propagation of this cancer. In agreement with this, several studies have postulated that brachyury represents a novel discriminating biomarker for chordomas. In particular, when combined with cytokeratin staining, the specificity for chordoma detection achieves 100 % [177, 191, 192].
Chordomas were also identified on the basis of their immunoreactivity for the S-100 protein, the epithelial MUC1 membrane glycoprotein and cytokeratins. Another genetic marker of chordomas is chondroitin sulfate proteoglycan 4 (CSPG4 or NG2/MCSP), which has been also targeted for immunotherapy. However, this antigen is expressed only by a fraction (62 %) of these tumors [193] and may lead to false negative diagnoses. Additional studies underlined the importance of some other notochord-related factors (such as Shh, Wnt, galectin-3 and NCAM) in the pathophysiology of chordoma. For example, the Wnt signaling pathway and the closely related cadherins appear to play a role in different cellular processes that take place in chordomas (for review, see [194]). Moreover, Shh has been found to be overexpressed in some clinical cases of chordoma, although Shh should not be considered a specific and selective marker for this tumor as anti-Shh antibodies also react in 50 % of the notochordal cases and in 81 % of chondrosarcoma cases [195]. In addition, the transcription factor Sox9, which is required for the maintenance of notochord integrity [196], is also expressed in chondrosarcoma samples [191]. These limitations underline the need for the improvement of chordoma diagnosis with new and more distinctive chordoma markers.
In addition, a gene expression profile of human chordoma samples revealed that different genes involved in extracellular matrix synthesis and control were upregulated [197]. These findings suggest that the inhibition of matrix-modifying proteases may prevent the local growth and distant spread of these tumors, by inhibiting the escape of cells from the extracellular matrix. Moreover, blocking proteins important for cell mobilization and cell–matrix interaction may be a reasonable alternative approach to the conventional therapeutic treatments.
In spite of these diverse findings, genetic studies did not reveal mutations that can be therapeutically targeted in chordomas. Studies of human chordoma samples have suggested activation of the EGFR-PI3 K-AKT-mTOR pathway [198, 199], and treatment of the chordoma cell line U-CH1 with mTORC1 and PI3 K inhibitors induces apoptosis [193]. Clinical trials have been carried out with a variety of receptor tyrosine kinase inhibitors, as well as with the mTOR inhibitor rapamycin [178]. Although partial responses have been observed in some patients, there is a pressing need for high-throughput preclinical models to direct targeted therapies in patients with this relatively rare tumor. Recently, it was reported the first in vivo genetic model for chordoma, based on stable notochord-specific expression of H-RasVal12 in zebrafish [200]. This model generates a reproducible tumor phenotype, ultimately leading to larval death. These tumors are highly similar to the human chordoma based on histological, ultrastructural and immunohistochemical features. The rapid onset of the tumor phenotype in this zebrafish chordoma model is well suited for screening for new pharmacological agents that can suppress tumor proliferation or induce tumor cell death. Indeed, the mTORC1 inhibitor rapamycin was shown to delay the onset of tumor formation and improve survival of tumor-bearing fish. Consequently, the zebrafish model of chordoma could enable high-throughput screening of potential therapeutic agents for the treatment of this refractory cancer [200].
The nucleus pulposus is thought to be required for the maintenance of the structural integrity of intervertebral discs and is the first structure to be affected during disc degeneration [201]. In this clinical context, notochord cells are proposed to display an important protective role, since in vitro and in vivo studies have suggested that notochord cells may represent the most effective cell population for repopulating the degenerate nucleus pulposus. Therefore, notochord cells may be the ideal type of cells able to repair a degenerated disc. Moreover, previous in vitro studies have highlighted the stimulatory effect of the conditioned medium of notochord cells on native intervertebral discs [202, 203]. A recent study pointed out the effect of notochord cell-derived conditioned media on mesenchymal stem cell differentiation toward a potentially nucleus pulposus-like phenotype, with some characteristics of the developing intervertebral discs [204]. These findings have important implications to understand fundamental biological processes acting on this complex structure not only in disc homeostasis, but more importantly in intervertebral disc degeneration and back pain.
Concluding remarks
The notochord is one of the earliest distinguishable hallmarks of the developing vertebrate embryo, directing essential patterning and structural activities. A broad number of studies provided insights into the molecular and mechanistic processes involved in notochord formation and homeostasis during embryonic development. Overall, these findings reinforce the concept that structure and function of the notochord are strictly linked, highlighting the signals that the peri-notochordal BM imparts to notochord cells. Besides providing valuable information on vertebrate biology and development, the available data indicate that the study of notochord development is helpful for throwing light on the origin and pathogenesis of chordoma, a rare and malignant bone cancer that originates from notochord remnants. The increasing knowledge on the genetic markers and molecular targets of chordomas is of high interest for devising new and effective therapies for this type of malignant cancer. We also discussed the role of notochord cells in forming the nucleus pulposus portion of the intervertebral discs, a feature that makes notochord cells a promising target for clinical approaches aimed at intervertebral disc repair and back pain treatment.
Acknowledgments
The authors are supported by grants from the University of Padova and the Italian Ministry of University and Research. We wish to thank Enrico Moro for the helpful comments and critical reading and Giacomo Cavaliere for the expert image assistance.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kowalevsky A. Entwicklungsgeschichte der einfachen Ascidien. Mem l’Acad St Petersbourg Ser. 1866;7:11–19. [Google Scholar]
- 2.Kowalevsky A. Entwicklungsgeschichte des Amphioxus lanceolatus. Mem l’Acad St Petersbourg Ser. 1866;7:1–17. [Google Scholar]
- 3.Satoh N, Tagawa K, Takahashi H. How was the notochord born? Evol Dev. 2012;14:56–75. doi: 10.1111/j.1525-142X.2011.00522.x. [DOI] [PubMed] [Google Scholar]
- 4.Satoh N, Rokhsar D, Nishikawa T. Chordate evolution and the three-phylum system. Proc Biol Sci. 2014;281:20141729. doi: 10.1098/rspb.2014.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hejnol A, Lowe CJ. Animal evolution: Stiff or squishy notochord origins? Curr Biol. 2014;24:R1131–R1133. doi: 10.1016/j.cub.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 6.Lauri A, Brunet T, Handberg-Thorsager M, Fischer A, Simakov O, Steinmetz P, Tomer R, Keller PJ, Arendt D. Development of the annelid axochord: Insight into notochord evolution. Science. 2014;345:1365–1368. doi: 10.1126/science.1253396. [DOI] [PubMed] [Google Scholar]
- 7.Harlan R, Gerhart J. Formation and function of Spemann’s organizer. Anuu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 8.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. Roux’s Arch Entw Mech. 1924;100:599–638. [PubMed] [Google Scholar]
- 9.Shih J, Fraser SE. Characterizing the zebrafish organizer: microsurgical analysis at the early-shield stage. Development. 1996;122:1313–1322. doi: 10.1242/dev.122.4.1313. [DOI] [PubMed] [Google Scholar]
- 10.Saúde L, Woolley K, Martin P, Driever W, Stemple DL. Axis-inducing activities and cell fates of the zebrafish organizer. Development. 2000;127:3407–3417. doi: 10.1242/dev.127.16.3407. [DOI] [PubMed] [Google Scholar]
- 11.Yan YL, Hatta K, Riggleman B, Postlethwait JH. Expression of a type II collagen gene in the zebrafish embryonic axis. Dev Dyn. 1995;203:363–376. doi: 10.1002/aja.1002030308. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, Chitnis AB, Matsumoto K, Crump JG, Hozumi K, Yonemura S, Kawakami K, Itoh M. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development. 2010;137:2527–2537. doi: 10.1242/dev.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leeson TS, Leeson CR. Observations on the histochemistry and fine structure of the notochord in rabbit embryos. J Anat. 1958;92:278–285. [PMC free article] [PubMed] [Google Scholar]
- 14.Bancroft M, Bellairs R. The development of the notochord in the chick embryo, studied by scanning and transmission electron microscopy. J Embryol Exp Morphol. 1976;35:383–401. [PubMed] [Google Scholar]
- 15.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 16.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200:667–679. doi: 10.1083/jcb.201212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue S. Ultrastructure of basement membranes. Int Rev Cytol. 1989;117:57–98. doi: 10.1016/s0074-7696(08)61334-0. [DOI] [PubMed] [Google Scholar]
- 18.Damjanov I. Heterogeneity of basement membranes in normal and pathologically altered tissues. Virchowa Arch A Pathol Anat Histopathol. 1990;416:185–188. doi: 10.1007/BF01678976. [DOI] [PubMed] [Google Scholar]
- 19.Erickson AC, Couchman J. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291–1306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 20.Timpl R, Brown JC. Supramolecular assembly of basement membranes. BioEssays. 1996;18(2):123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 21.Camón J, Degollada E, Verdú J. Ultrastructural aspects of the production of extracellular matrix components by the chick embryonic notochord in vitro. Acta Anat (Basel) 1990;137:114–123. doi: 10.1159/000146869. [DOI] [PubMed] [Google Scholar]
- 22.Parsons MJ, Pollard SM, Saude L, Feldman B, Coutinho P, Hirst EM, Stemple DL. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–3146. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- 23.Scott A, Stemple DL. Zebrafish notochordal basement membrane: signaling and structure. Curr Top Dev Biol. 2005;65:229–253. doi: 10.1016/S0070-2153(04)65009-5. [DOI] [PubMed] [Google Scholar]
- 24.Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–2512. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- 25.Pagnon-Minot A, Malbouyres M, Haftek-Terrau Z, Kim HR, Sasaki T, Thisse C, Thisse B, Ingham PW, Ruggiero F, Le Guellec D. Collagen XV, a novel factor in zebrafish notochord differentiation and muscle development. Developmental Biology. 2008;316:21–35. doi: 10.1016/j.ydbio.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Mangos S, Lam P, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech. 2010;3:354–365. doi: 10.1242/dmm.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corallo D, Schiavinato A, Trapani V, Moro E, Argenton F, Bonaldo P. Emilin3 is required for notochord sheath integrity and interacts with Scube2 to regulate notochord-derived Hedgehog signals. Development. 2013;140:4594–4601. doi: 10.1242/dev.094078. [DOI] [PubMed] [Google Scholar]
- 28.Buisson N, Sirour C, Moreau N, Denker E, Le Bouffant R, Goullancourt A, Darribère T, Bello V. An adhesome comprising laminin, dystroglycan and myosin IIA is required during notochord development in Xenopus laevis . Development. 2014;141:4569–4579. doi: 10.1242/dev.116103. [DOI] [PubMed] [Google Scholar]
- 29.Theiler K. Vertebral malformations. Adv Anat Embryol Cell Biol. 1988;112:1–99. doi: 10.1007/978-3-642-73775-6. [DOI] [PubMed] [Google Scholar]
- 30.von Dassow G, Schmidt JE, Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev. 1993;7:355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]
- 31.Talbot WS, Trevarrow B, Halpern ME, Melby AE, Farr G, Postlethwait JH, Jowett T, Kimmel CB, Kimelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- 32.Knezevic V, Ranson M, Mackem S. The organizer-associated chick homeobox gene, Gnot1, is expressed before gastrulation and regulated synergistically by activin and retinoic acid. Dev Biol. 1995;171:458–470. doi: 10.1006/dbio.1995.1296. [DOI] [PubMed] [Google Scholar]
- 33.Halpern ME, Thisse C, Ho RK, Thisse B, Riggleman B, Trevarrow B, Weinberg ES, Postlethwait JH, Kimmel CB. Cell-autonomous shift from axial to paraxial mesodermal development in zebrafish floating head mutants. Development. 1995;121:4257–4264. doi: 10.1242/dev.121.12.4257. [DOI] [PubMed] [Google Scholar]
- 34.Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature. 1989;337:358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- 35.Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- 36.Gont LK, Fainsod A, Kim SH, De Robertis EM. Overexpression of the homeobox gene Xnot-2 leads to notochord formation in Xenopus. Dev Biol. 1996;174:174–178. doi: 10.1006/dbio.1996.0061. [DOI] [PubMed] [Google Scholar]
- 37.Yasuo H, Lemaire P. Role of Goosecoid, Xnot and Wnt antagonists in the maintenance of the notochord genetic programme in Xenopus gastrulae. Development. 2001;128:3783–3793. doi: 10.1242/dev.128.19.3783. [DOI] [PubMed] [Google Scholar]
- 38.Abdelkhalek HA, Beckers A, Schuster-Gossler K, Pavlova MN, Burkhardt H, Lickert H, Rossant J, Reinhardt R, Schalkwyk LC, Müller I, Herrmann BG, Ceolin M, Rivera-Pomar R, Gossler A. The mouse homeobox gene Not is required for caudal notochord development and affected by the truncate mutation. Genes Dev. 2004;18:1725–1736. doi: 10.1101/gad.303504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev. 1998;12:2345–2353. doi: 10.1101/gad.12.15.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, Schier AF, Driever W, Hirano T, Talbot WS, Solnica-Krezel L. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development. 1999;126:1427–1438. doi: 10.1242/dev.126.7.1427. [DOI] [PubMed] [Google Scholar]
- 41.Odenthal J, Haffter P, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- 42.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 43.Chiu CH, Chou CW, Takada S, Liu YW. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS ONE. 2012;7:e43040. doi: 10.1371/journal.pone.0043040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin α5β1, fibronectin, and tissue geometry. Dev Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- 45.Davidson LA, Marsden M, Keller R, Desimone DW. Integrin α5β1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 46.George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- 47.Georges-Labouesse EN, George EL, Rayburn H, Hynes RO. Mesodermal development in mouse embryos mutant for fibronectin. Dev Dyn. 1996;207:145–156. doi: 10.1002/(SICI)1097-0177(199610)207:2<145::AID-AJA3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 48.Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- 49.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 50.Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- 51.Schulte-Merker S, van Eeden FJ, Halpern ME, Kimmel CB, Nüsslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- 52.Kispert A, Herrmann BG. Immunohistochemical analysis of the Brachyury protein in wild-type and mutant mouse embryos. Dev Biol. 1994;161:179–193. doi: 10.1006/dbio.1994.1019. [DOI] [PubMed] [Google Scholar]
- 53.Kispert A, Ortner H, Cooke J, Herrmann BG. The chick Brachyury gene: developmental expression pattern and response to axial induction by localized activin. Dev Biol. 1995;168:406–415. doi: 10.1006/dbio.1995.1090. [DOI] [PubMed] [Google Scholar]
- 54.Kispert A, Hermann BG. The Brachyury gene encodes a novel DNA binding protein. EMBO J. 1993;12:4898–4899. doi: 10.1002/j.1460-2075.1993.tb06179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conlon FL, Sedgwick SG, Weston KM, Smith JC. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development. 1996;122:2427–2435. doi: 10.1242/dev.122.8.2427. [DOI] [PubMed] [Google Scholar]
- 56.Wilkinson DG, Bhatt S, Hermann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 57.Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- 58.Schulte-Merker S, Ho RK, Herrmann BG, Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- 59.Wilson V, Manson L, Skarnes WC, Beddington RS. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development. 1995;121:877–886. doi: 10.1242/dev.121.3.877. [DOI] [PubMed] [Google Scholar]
- 60.Melby AE, Kimelman D, Kimmel CB. Spatial regulation of floating head expression in the developing notochord. Dev Dyn. 1997;209:156–165. doi: 10.1002/(SICI)1097-0177(199706)209:2<156::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 61.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 62.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pollard SM, Parsons MJ, Kamei M, Kettleborough RNW, Thomas KA, Van Pham N, Bae M, Scott A, Weinstein BM, Stemple DL. Essential and overlapping roles for laminin α chains in notochord and blood vessel formation. Dev Biol. 2006;289:64–76. doi: 10.1016/j.ydbio.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 64.Fratzl P. Collagen: structure and mechanics. New York: Springer; 2008. [Google Scholar]
- 65.Gansner JM, Gitlin JD. Essential role for the alpha 1 chain of type VIII collagen in zebrafish notochord formation. Dev Dyn. 2011;237:3715–3726. doi: 10.1002/dvdy.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christiansen HE, Lang MR, Pace JM, Parichy DM. Critical early roles for col27a1a and col27a1b in zebrafish notochord morphogenesis, vertebral mineralization and post-embryonic axial growth. PLoS ONE. 2009;4:e8481. doi: 10.1371/journal.pone.0008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gansner JM, Mendelsohn BA, Hultman KA, Johnson SL, Gitlin JD. Essential role of lysyl oxidases in notochord development. Dev Biol. 2007;307:202–213. doi: 10.1016/j.ydbio.2007.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Menkes JH. Kinky hair disease: twenty five years later. Brain Dev. 1988;10:77–79. doi: 10.1016/s0387-7604(88)80074-3. [DOI] [PubMed] [Google Scholar]
- 70.Madsen EC, Gitlin JD. Zebrafish mutants calamity and catastrophe define critical pathways of gene-nutrient interactions in developmental copper metabolism. PLoS Genet. 2008;4:e1000261. doi: 10.1371/journal.pgen.1000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gansner JM, Madsen EC, Mecham RP, Gitlin JD. Essential role for fibrillin-2 in zebrafish notochord and vascular morphogenesis. Dev Dyn. 2008;237:2844–2861. doi: 10.1002/dvdy.21705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 73.Coutinho P, Parsons MJ, Thomas KA, Hirst EM, Saúde L, Campos I, Williams PH, Stemple DL. Differential requirements for COPI transport during vertebrate early development. Dev Cell. 2004;7:547–558. doi: 10.1016/j.devcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 74.Nickel W, Brügger B, Wieland FT. Vesicular transport: the core machinery of COPI recruitment and budding. J Cell Sci. 2002;115:3235–3240. doi: 10.1242/jcs.115.16.3235. [DOI] [PubMed] [Google Scholar]
- 75.Kelsh RN, Brand M, Jiang YJ, Heisenberg CP, Lin S, Haffter P, Odenthal J, Mullins MC, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Kane DA, Warga RM, Beuchle D, Vogelsang L, Nüsslein-Volhard C. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–389. doi: 10.1242/dev.123.1.369. [DOI] [PubMed] [Google Scholar]
- 76.Ekker SC, Larson JD. Morphant technology in model developmental systems. Genesis. 2001;30:89–93. doi: 10.1002/gene.1038. [DOI] [PubMed] [Google Scholar]
- 77.Pickart MA, Klee EW, Nielsen AL, Sivasubbu S, Mendenhall EM, Bill BR, Chen E, Eckfeldt CE, Knowlton M, Robu ME, et al. Genome-wide reverse genetics framework to identify novel functions of the vertebrate secretome. PLoS ONE. 2006;1:e104. doi: 10.1371/journal.pone.0000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA, Lawson ND. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev Cell. 2015;32:97–108. doi: 10.1016/j.devcel.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cleaver O, Krieg PA. Notochord patterning of the endoderm. Dev Biol. 2001;234:1–12. doi: 10.1006/dbio.2001.0214. [DOI] [PubMed] [Google Scholar]
- 81.Briscoe J, Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin Cell Dev Biol. 1999;10:353–362. doi: 10.1006/scdb.1999.0295. [DOI] [PubMed] [Google Scholar]
- 82.Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development Suppl. 1991;2:105–122. [PubMed] [Google Scholar]
- 83.Yamada T, Placzek M, Tanaka H, Dodd J, Jessell TM. Control of cell pattern in the developing nervous system: Polarizing activity of the floor plate and notochord. Cell. 1990;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 84.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 85.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 86.Martí E, Takada R, Bumcrot DA, Sasaki H, McMahon AP. Distribution of Sonic hedgehog peptides in the developing chick and mouse embryo. Development. 1995;121:2537–2547. doi: 10.1242/dev.121.8.2537. [DOI] [PubMed] [Google Scholar]
- 87.Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- 88.Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol. 1997;62:451–466. [PubMed] [Google Scholar]
- 89.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1992;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 90.Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1992;117:205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- 91.Yamada T, Pfaff SL, Edlund T, Jessell TM. Control of cell pattern in the neural tube: motor neuron induction by diffusible factors from notochord and floor plate. Cell. 1992;73:673–686. doi: 10.1016/0092-8674(93)90248-o. [DOI] [PubMed] [Google Scholar]
- 92.Chamberlain CE, Jeong J, Guo C, Allen BL, McMahon AP. Notochord-derived Shh concentrates in close association with the apically positioned basal body in neural target cells and forms a dynamic gradient during neural patterning. Development. 2008;135:1097–1106. doi: 10.1242/dev.013086. [DOI] [PubMed] [Google Scholar]
- 93.Odenthal J, van Eeden FJM, Haffter P, Ingham PW, Nusslein-Volhard C. Two distinct cell populations in the floor plate of the zebrafish are induced by different pathways. Dev Biol. 2000;219:350–363. doi: 10.1006/dbio.1999.9589. [DOI] [PubMed] [Google Scholar]
- 94.Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strahle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- 95.Ribes V, Balaskas N, Sasai N, Cruz C, Dessaud E, Cayuso J, Tozer S, Yang LL, Novitch B, Marti E, Briscoe J. Distinct Sonic Hedgehog signaling dynamics specify floor plate and ventral neuronal progenitors in the vertebrate neural tube. Genes Dev. 2010;24:1186–1200. doi: 10.1101/gad.559910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liem KF, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1994;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 97.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1998;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 98.Tozer S, Le Dréau G, Marti E, Briscoe J. Temporal control of BMP signalling determines neuronal subtype identity in the dorsal neural tube. Development. 2013;140:1467–1474. doi: 10.1242/dev.090118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patten I, Placzek M. Opponent activities of Shh and BMP signaling during floor plate induction in vivo. Curr Biol. 2002;12:47–52. doi: 10.1016/s0960-9822(01)00631-5. [DOI] [PubMed] [Google Scholar]
- 100.Martinez-Morales JR, Barbas JA, Martí E, Bovolenta P, Edgar D, Rodriguez-Tébar A. Vitronectin is expressed in the ventral region of the neural tube and promotes the differentiation of motor neurons. Development. 1997;124:5139–5147. doi: 10.1242/dev.124.24.5139. [DOI] [PubMed] [Google Scholar]
- 101.Pons S, Trejo JL, Martinez-Morales JR, Martí E. Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development. 2001;128:1481–1492. doi: 10.1242/dev.128.9.1481. [DOI] [PubMed] [Google Scholar]
- 102.Gleiberman AS, Fedtsova NG, Rosenfeld MG. Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol. 1998;213:340–353. doi: 10.1006/dbio.1999.9386. [DOI] [PubMed] [Google Scholar]
- 103.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disk degeneration and chordoma formation. Dev Dyn. 2008;237:3953–3958. doi: 10.1002/dvdy.21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi KS, Harfe BD. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci USA. 2011;108:9484–9489. doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Choi KS, Lee C, Harfe BD. Sonic hedgehog in the notochord is sufficient for patterning of the intervertebral discs. Mech Dev. 2012;129:255–262. doi: 10.1016/j.mod.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- 107.Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 108.Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 109.Fan CM, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 110.Teillet M, Watanabe Y, Jeffs P, Duprez D, Lapointe F, Le Douarin NM. Sonic hedgehog is required for survival of both myogenic and chondrogenic somitic lineages. Development. 1998;125:2019–2030. doi: 10.1242/dev.125.11.2019. [DOI] [PubMed] [Google Scholar]
- 111.Buttitta L, Mo R, Hui CC, Fan CM. Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development. 2003;130:6233–6243. doi: 10.1242/dev.00851. [DOI] [PubMed] [Google Scholar]
- 112.Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell. 2001;106:781–792. [PubMed] [Google Scholar]
- 113.Borycki AG, Mendham L, Emerson CP. Control of somite patterning by Sonic hedgehog and its downstream signal response genes. Development. 1998;125:777–790. doi: 10.1242/dev.125.4.777. [DOI] [PubMed] [Google Scholar]
- 114.McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dockter JL. Sclerotome induction and differentiation. Curr Top Dev Biol. 2000;48:77–127. doi: 10.1016/s0070-2153(08)60755-3. [DOI] [PubMed] [Google Scholar]
- 116.Ando T, Semba K, Suda H, Sei A, Mizuta H, Araki M, Abe K, Imai K, Nakagata N, Araki K, Yamamura K. The floor plate is sufficient for development of the sclerotome and spine without the notochord. Mech Dev. 2011;128:129–140. doi: 10.1016/j.mod.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 117.El-Magd MA, Allen S, McGonnell I, Mansour AA, Otto A, Patel K. Shh regulates chick Ebf1 gene expression in somite development. Gene. 2014;554:87–95. doi: 10.1016/j.gene.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 118.Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development. 1999;126:4053–4063. doi: 10.1242/dev.126.18.4053. [DOI] [PubMed] [Google Scholar]
- 119.Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein DJ, Emerson CP. Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev. 2002;16:114–126. doi: 10.1101/gad.940702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McDermott A, Gustafsson M, Elsam T, Hui CC, Emerson CP, Jr, Borycki AG. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development. 2005;132:345–357. doi: 10.1242/dev.01537. [DOI] [PubMed] [Google Scholar]
- 121.Münsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 122.Feng X, Adiarte EG, Devoto SH. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol. 2006;300:736–746. doi: 10.1016/j.ydbio.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 123.Hammond CL, Hinits Y, Osborn DP, Minchin JE, Tettamanti G, Hughes SM. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev Biol. 2007;302:504–521. doi: 10.1016/j.ydbio.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cairns DM, Sato ME, Lee PG, Lassar AB, Zeng L. A gradient of Shh establishes mutually repressing somitic cell fates induced by Nkx3.2 and Pax3. Dev Biol. 2008;323:152–165. doi: 10.1016/j.ydbio.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kahane N, Ribes V, Kicheva A, Briscoe J, Kalcheim C. The transition from differentiation to growth during dermomyotome-derived myogenesis depends on temporally restricted hedgehog signaling. Development. 2013;140:1740–1750. doi: 10.1242/dev.092726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–1181. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 127.Ingham PW, Kim HR. Hedgehog signalling and the specification of muscle cell identity in the zebrafish embryo. Exp Cell Res. 2005;306:336–342. doi: 10.1016/j.yexcr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 128.Hatta K, Bremiller R, Westerfield M, Kimmel CB. Diversity of expression of engrailed-like antigens in zebrafish. Development. 1991;112:821–832. doi: 10.1242/dev.112.3.821. [DOI] [PubMed] [Google Scholar]
- 129.Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, Driever W. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–128. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- 130.Coutelle O, Blagden CS, Hampson R, Halai C, Rigby PW, Hughes SM. Hedgehog signalling is required for maintenance of myf5 and myoD expression and timely terminal differentiation in zebrafish adaxial myogenesis. Dev Biol. 2001;236:136–150. doi: 10.1006/dbio.2001.0193. [DOI] [PubMed] [Google Scholar]
- 131.Goldstein AM, Fishman MC. Notochord regulates cardiac lineage in zebrafish embryos. Dev Biol. 1998;201:247–252. doi: 10.1006/dbio.1998.8976. [DOI] [PubMed] [Google Scholar]
- 132.Danos MC, Yost HJ. Role of notochord in specification of cardiac left–right orientation in zebrafish and Xenopus. Dev Biol. 1996;177:96–103. doi: 10.1006/dbio.1996.0148. [DOI] [PubMed] [Google Scholar]
- 133.Lohr JL, Danos MC, Yost HJ. Left–right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–1472. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- 134.Melloy PG, Ewart JL, Cohen MF, Desmond ME, Kuehn MR, Lo CW. No turning, a mouse mutation causing left–right and axial patterning defects. Dev Biol. 1998;193:77–89. doi: 10.1006/dbio.1997.8787. [DOI] [PubMed] [Google Scholar]
- 135.Wong KS, Rehn K, Palencia-Desai S, Kohli V, Hunter W, Uhl JD, Rost MS, Sumanas S. Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish. Dev Biol. 2012;361:377–391. doi: 10.1016/j.ydbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 136.Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Res Dis. 1989;140:1097–1103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- 137.Risau W, Flamme I. Vasculogenesis. Annu Rev Cell Dev Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. [DOI] [PubMed] [Google Scholar]
- 138.Sumoy L, Keasey JB, Dittman TD, Kimelman D. A role for notochord in axial vascular development revealed by analysis of phenotype and the expression of VEGR-2 in zebrafish flh and ntl mutant embryos. Mech Dev. 1997;63:15–27. doi: 10.1016/s0925-4773(97)00671-0. [DOI] [PubMed] [Google Scholar]
- 139.Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. [DOI] [PubMed] [Google Scholar]
- 140.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 141.Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- 142.Tripathi P, Guo Q, Wang Y, Coussens M, Liapis H, Jain S, Kuehn MR, Capecchi MR, Chen F. Midline signaling regulates kidney positioning but not nephrogenesis through Shh. Dev Biol. 2010;340:518–527. doi: 10.1016/j.ydbio.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murashima A, Akita H, Okazawa M, Kishigami S, Nakagata N, Nishinakamura R, Yamada G. Midline-derived Shh regulates mesonephric tubule formation through the paraxial mesoderm. Dev Biol. 2014;386:216–226. doi: 10.1016/j.ydbio.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 145.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cleaver O, Seufert DW, Krieg PA. Endoderm patterning by the notochord: development of the hypochord in Xenopus. Development. 2000;127:869–879. doi: 10.1242/dev.127.4.869. [DOI] [PubMed] [Google Scholar]
- 147.Fausett SR, Brunet LJ, Klingensmith J. BMP antagonism by Noggin is required in presumptive notochord cells for mammalian foregut morphogenesis. Dev Biol. 2014;391:111–124. doi: 10.1016/j.ydbio.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 148.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 149.Anderson C, Thorsteinsdóttir S, Borycki AG. Sonic hedgehog-dependent synthesis of laminin alpha1 controls basement membrane assembly in the myotome. Development. 2009;136:3495–3504. doi: 10.1242/dev.036087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Costello I, Biondi CA, Taylor JM, Bikoff EK, Robertson EJ. Smad4-dependent pathways control basement membrane deposition and endodermal cell migration at early stages of mouse development. BMC Dev Biol. 2009;9:54. doi: 10.1186/1471-213X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- 152.Dolez M, Nicolas JF, Hirsinger E. Laminins, via heparan sulfate proteoglycans, participate in zebrafish myotome morphogenesis by modulating the pattern of Bmp responsiveness. Development. 2011;138:97–106. doi: 10.1242/dev.053975. [DOI] [PubMed] [Google Scholar]
- 153.Arteaga-Solis E, Gayraud B, Lee SY, Shum L, Sakai L, Ramirez F. Regulation of limb patterning by extracellular microfibrils. J Cell Biol. 2001;154:275–281. doi: 10.1083/jcb.200105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 2012;26:1312–1325. doi: 10.1101/gad.191866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tukachinsky H, Kuzmickas RP, Jao CY, Liu J, Salic A. Dispatched and scube mediate the efficient secretion of the cholesterol-modified hedgehog ligand. Cell Rep. 2012;2:308–320. doi: 10.1016/j.celrep.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schaffer J. In von Mollendorff’s “Handbuch der Mikroskopischen Anatomie des Menschen”. Berlin: Springer; 1910. pp. 31–44. [Google Scholar]
- 158.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 159.Aszodi A, Chan D, Hunziker E, Bateman JF, Fassler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haga Y, Dominique VJ, 3rd, Du SJ. Analyzing notochord segmentation and intervertebral disc formation using the twhh:gfp transgenic zebrafish model. Transgenic Res. 2009;18:669–683. doi: 10.1007/s11248-009-9259-y. [DOI] [PubMed] [Google Scholar]
- 161.Lefebvre V. Toward understanding the functions of the two highly related Sox5 and Sox6 genes. J Bone Miner Metab. 2002;20:121–130. doi: 10.1007/s007740200017. [DOI] [PubMed] [Google Scholar]
- 162.Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- 163.Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- 164.Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:5399–5408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 165.Grotmol S, Kryvi H, Nordvik K, Totland GK. Notochord segmentation may lay down the pathway for the development of the vertebral bodies in the Atlantic salmon. Anat Embryol (Berl) 2003;207:263–272. doi: 10.1007/s00429-003-0349-y. [DOI] [PubMed] [Google Scholar]
- 166.Dale RM, Topczewski J. Identification of an evolutionarily conserved regulatory element of the zebrafish col2a1a gene. Dev Biol. 2011;357:518–531. doi: 10.1016/j.ydbio.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–880. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- 168.Grey RS, Wilm TP, Smith J, Bagnat M, Dale RM, Topczewski J, Johnson SL, Solnica-Krezel L. Loss of col8a1a function during zebrafish embryogenesis results in congenital vertebral malformations. Dev Biol. 2014;386:72–85. doi: 10.1016/j.ydbio.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Butler WF. Comparative anatomy and development of the mammalian disc. In: Gosh P, editor. The biology of the intervertebral disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- 170.Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur Spine J. 2014;23:1803–1814. doi: 10.1007/s00586-014-3305-z. [DOI] [PubMed] [Google Scholar]
- 171.Frick SL, Hanley EN, Meyer RA, JrRamp WK, JrChapman TM. Lumbar intervertebral disc transfer. A canine study. Spine. 1994;19:1826–1834. doi: 10.1097/00007632-199408150-00006. [DOI] [PubMed] [Google Scholar]
- 172.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 173.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 174.Hutton WC, Ganey TM, Elmer WA, Kozlowska E, Ugbo JL, Doh ES, Whitesides TE., Jr Does long-term compressive loading on the intervertebral disc cause degeneration? Spine. 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 175.Kadoya K, Kotani Y, Abumi K, Takada T, Shimamoto N, Shikinami Y, Kadosawa T, Kaneda K. Biomechanical and morphologic evaluation of a three-dimensional fabric sheep artificial intervertebral disc: in vitro and in vivo analysis. Spine. 2001;26:1562–1569. doi: 10.1097/00007632-200107150-00012. [DOI] [PubMed] [Google Scholar]
- 176.Kawchuk GN, Kaigle AM, Holm SH, Rod FO, Ekstrom L, Hansson T. The diagnostic performance of vertebral displacement measurements derived from ultrasonic indentation in an in vivo model of degenerative disc disease. Spine. 2001;26:1348–1355. doi: 10.1097/00007632-200106150-00018. [DOI] [PubMed] [Google Scholar]
- 177.Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209:157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 178.Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69–e76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 179.Persson S, Kindblom LG, Angervall L. Classical and chondroid chordoma: a light-microscopic, histochemical, ultrastructural and immunohistochemical analysis of the various cell types. Pathol Res Pract. 1991;187:828–838. doi: 10.1016/S0344-0338(11)80579-0. [DOI] [PubMed] [Google Scholar]
- 180.Yamaguchi T, Suzuki S, Ishiiwa H, Ueda Y. Intraosseous benign notochordal cell tumours: overlooked precursors of classical chordomas? Histopathology. 2004;44:597–602. doi: 10.1111/j.1365-2559.2004.01877.x. [DOI] [PubMed] [Google Scholar]
- 181.Takahiko N, Iwamoto Y, Shinohara N, Chuman H, Fukui M, Tsuneyoshi M. Cytokeratin subtyping in chordomas and the fetal notochord: an immunohistochemical analysis of aberrant expression. Mod Pathol. 1997;10:545–551. [PubMed] [Google Scholar]
- 182.Salisbury JR. The pathology of the notochord: review article. J Pathol. 1993;171:153–155. doi: 10.1002/path.1711710404. [DOI] [PubMed] [Google Scholar]
- 183.Mirra JM, Nelson SD, Della Rocca C, Mertens F. Notochordal tumours. In: Fletcher KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. pp. 315–317. [Google Scholar]
- 184.Showell C, Binder O, Conlon FL. T-box genes in embryogenesis. A review. Dev Dyn. 2004;229:202–218. doi: 10.1002/dvdy.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yang XR, Ng D, Alcorta DA, Liebsch NJ, Sheridan E, Li S, Goldstein AM, Parry DM, Kelley MJ. T (brachyury) gene duplication confers major susceptibility to familial chordoma. Nat Genet. 2009;41:1176–1178. doi: 10.1038/ng.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Presneau N, Shalaby A, Ye H, Pillay N, Halai D, Idowu B, Tirabosco R, Whitwell D, Jacques TS, Kindblom LG, et al. Role of the transcription factor T (brachyury) in the pathogenesis of sporadic chordoma: a genetic and functional-based study. J Pathol. 2011;223:327–335. doi: 10.1002/path.2816. [DOI] [PubMed] [Google Scholar]
- 187.Pillay N, Plagnol V, Tarpey PS, Lobo SB, Presneau N, Szuhai K, Halai D, Berisha F, Cannon SR, Mead S, Kasperaviciute D, Palmen J, Talmud PJ, Kindblom LG, Amary MF, Tirabosco R, Flanagan AM. A common single-nucleotide variant in T is strongly associated with chordoma. Nat Genet. 2012;44:1185–1187. doi: 10.1038/ng.2419. [DOI] [PubMed] [Google Scholar]
- 188.Nelson AC, Pillay N, Henderson S, Presneau N, Tirabosco R, Halai D, Berisha F, Flicek P, Stemple DL, Stern CD, Wardle FC, Flanagan AM. An integrated functional genomics approach identifies the regulatory network directed by brachyury (T) in chordoma. J Pathol. 2012;228(3):274–285. doi: 10.1002/path.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hsu W, Mohyeldin A, Shah SR, Ap Rhys CM, Johnson LF, Sedora-Roman NI, Kosztowski TA, Awad OA, et al. Generation of chordoma cell line JHC7 and the identification of Brachyury as a novel molecular target. J Neurosurg. 2011;115:760–769. doi: 10.3171/2011.5.JNS11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–544. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Oakley GJ, Fuhrer K, Seethala RR. Brachyury, SOX-9, and podoplanin, new markers in the skull base chordoma vs chondrosarcoma differential: a tissue microarray-based comparative analysis. Mod Pathol. 2008;21:1461–1469. doi: 10.1038/modpathol.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sangoi AR, Karamchandani J, Lane B, Higgins JP, Rouse RV, Brooks JD, McKenney JK. Specificity of brachyury in the distinction of chordoma from clear cell renal cell carcinoma and germ cell tumors: a study of 305 cases. Mod Pathol. 2011;24:425–429. doi: 10.1038/modpathol.2010.196. [DOI] [PubMed] [Google Scholar]
- 193.Schwab J, Antonescu C, Boland P, Healey J, Rosenberg A, Nielsen P, Iafrate J, Delaney T, Yoon S, Choy E, Harmon D, et al. Combination of PI3K/mTOR inhibition demonstrates efficacy in human chordoma. Anticancer Res. 2009;29:1867–1871. [PubMed] [Google Scholar]
- 194.Yakkioui Y, van Overbeeke JJ, Santegoeds R, van Engeland M, Temel Y. Chordoma: the entity. Biochim Biophys Acta. 2014;1846:655–669. doi: 10.1016/j.bbcan.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 195.Cates JM, Itani DM, Coffin CM, Harfe BD. The sonic hedgehog pathway in chordoid tumours. Histopathology. 2010;56:978–979. doi: 10.1111/j.1365-2559.2010.03572.x. [DOI] [PubMed] [Google Scholar]
- 196.Barrionuevo F, Taketo MM, Scherer G, Kispert A. Sox9 is required for notochord maintenance in mice. Dev Biol. 2006;295:128–140. doi: 10.1016/j.ydbio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 197.Schwab JH, Boland PJ, Agaram NP, Socci ND, Guo T, O’Toole GC, Wang X, Ostroumov E, Hunter CJ, Block JA, Doty S, Ferrone S, Healey JH, Antonescu CR. Chordoma and chondrosarcoma gene profile: implications for immunotherapy. Cancer Immunol Immunother. 2009;58:339–349. doi: 10.1007/s00262-008-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Han S, Polizzano C, Nielsen GP, Hornicek FJ, Rosenberg AE, Ramesh V. Aberrant hyperactivation of akt and Mammalian target of rapamycin complex 1 signaling in sporadic chordomas. Clin Cancer Res. 2009;15:1940–1946. doi: 10.1158/1078-0432.CCR-08-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Presneau N, Shalaby A, Idowu B, Gikas P, Cannon SR, Gout I, Diss T, Tirabosco R, Flanagan AM. Potential therapeutic targets for chordoma: PI3 K/AKT/TSC1/TSC2/mTOR pathway. Br J Cancer. 2009;100:1406–1414. doi: 10.1038/sj.bjc.6605019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Burger A, Vasilyev A, Tomar R, Selig MK, Nielsen GP, Peterson RT, Drummond IA, Haber DA. A zebrafish model of chordoma initiated by notochord-driven expression of HRASV12. Dis Model Mech. 2014;7:907–913. doi: 10.1242/dmm.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–97. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine. 2006;31:1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- 203.Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J. Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine. 2006;31:873–882. doi: 10.1097/01.brs.0000209302.00820.fd. [DOI] [PubMed] [Google Scholar]
- 204.Korecky CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]