Abstract
Several lines of evidence support the relevance of microRNAs in both adrenocortical and adrenomedullary (pheochromocytomas) tumors. Significantly differentially expressed microRNAs have been described among benign and malignant adrenocortical tumors and different forms of pheochromocytomas that might affect different pathogenic pathways. MicroRNAs can be exploited as markers of malignancy or disease recurrence. Besides tissue microRNAs, novel data show that microRNAs are released in body fluids, and blood-borne microRNAs can be envisaged as minimally invasive markers of malignancy or prognosis. MicroRNAs might even serve as treatment targets that could expand the rather-limited therapeutic repertoire in the field of adrenal tumors. In this review, we present a critical synopsis of the recent observations made in the field of adrenal tumor-associated microRNAs regarding their pathogenic, diagnostic, and potential therapeutic relevance.
Keywords: Biomarker, Pathway, Cancer, Treatment
Introduction
Adrenal tumors are common with an average frequency of 2–3 % in autopsy series [1]. Most of these tumors are clinically silent and incidentally discovered benign adrenocortical tumors [1]. Hormone-producing adrenocortical adenomas (cortisol—Cushing’s syndrome, aldosterone—primary aldosteronism) and catecholamine-secreting adrenomedullary pheochromocytomas (PCC), however, result in significant morbidity and mortality. Malignant tumors of the adrenal, adrenocortical cancer (ACC), and malignant pheochromocytoma are rare [2, 3]. The prognosis of ACC is poor in advanced stages: 5-year survival in stage 3 is 50 %, and only 13 % in stage 4 [2]. The five-year survival of malignant PCC varies between 20 and 50 % [3].
The pathogenesis of sporadic adrenocortical tumors is poorly elucidated, and several questions are to be answered in PCC tumorigenesis as well. There are several challenges in the clinical management of these tumors including the lack of reliable preoperative plasma or serum markers of malignancy or recurrence. The histological diagnosis of malignancy in adrenocortical tumors is problematic, but it is not feasible in PCC at all where malignancy can only be established by the appearance of metastases [2–4]. Besides surgical removal, there are few effective medical treatment options for advanced metastatic adrenocortical and adrenomedullary tumors [2–4].
Since the discovery of RNA interference and the subsequent description of microRNAs as their endogenous mediators, a vast array of experimental findings underline the relevance of microRNAs and their pathways in various human tumors [5, 6]. MicroRNAs are non-protein-coding small RNA molecules encoded by distinct genes that undergo a sophisticated maturation process and their mature single-stranded forms specifically bind the 3′ untranslated region of messenger RNAs (mRNA). Cytoplasmic microRNAs inhibit mRNA translation or induce their degradation and thereby constitute a very specific way of posttranscriptional gene expression regulation forming part of the epigenetic machinery [6]. MicroRNAs are involved in the regulation of basic cell biological processes, immune regulation, ontogenesis, etc. [7]. Novel data show that besides posttranscriptional gene regulation exerted by cytoplasmic microRNAs, nuclear microRNAs are also involved in the regulation of gene transcription [8].
Overexpressed microRNAs in tumors relative to normal tissues have been termed oncogenes, whereas underexpressed microRNAs relative to normal tissues are regarded as tumor suppressors following the classical oncogene-tumor suppressor dichotomy [5, 6, 9]. Oncogenic microRNAs might contribute to tumorigenesis by targeting cellular tumor suppressor mRNAs, whereas tumor suppressor microRNAs target oncogenic mRNAs [9]. MicroRNAs act in a tissue-specific manner and therefore the same microRNA can function as a tumor suppressor in one tissue, but also as an oncogene in another [9].
Differentially expressed microRNAs can be exploited as markers of malignancy. This can be especially useful for the diagnosis of tumors, where histological analysis is difficult (e.g., diagnosis of differentiated thyroid tumors) [10]. Since microRNAs are surprisingly stable, their diagnostic applicability is greatly extended by the use of formalin-fixed paraffin-embedded (FFPE) archived tissue blocks [11]. Novel observations have revealed that microRNAs enter body fluids via the secretion of exosomes, microvesicles, or in complex with macromolecules like high-density lipoprotein and might exhibit features of hormones conveying gene expression information [12]. Blood-borne (plasma or serum) microRNAs might have great potential as minimally invasive biomarkers, and there are several data in various tumors supporting their clinical applicability [13]. Apart from their pathogenic and diagnostic relevance, microRNAs might also be envisaged as potential future targets of therapy [14].
The potential of miRNAs in the diagnosis and treatment of adrenal tumors remains to be exploited because of numerous unresolved issues associated with miRNA research. These include differences in microRNA profiling results established by different research groups and numerous technical issues. Different platforms (low-density polymerase chain reaction (PCR)-based arrays, microarrays, next-generation sequencing), statistical approaches, choice of reference genes, etc., might account for these discrepancies in addition to the different composition of patient cohorts. The target mRNAs for microRNAs are most easily identified by in silico target prediction approaches that must be validated experimentally [15]. Results of high-throughput analyses and microRNA-based pathways must also be validated by a different technique (e.g., real-time PCR) [16].
In this review, we present a synopsis of the recent observations made in the field of ACC- and PCC-associated microRNAs highlighting their relevance in tumorigenesis, diagnosis, and potential for therapy.
MicroRNAs in adrenocortical cancer
Several pathways have been shown to be implicated in ACC pathogenesis including insulin-like growth factor 2 (IGF2) signaling, the tumor suppressor P53, Wnt/β-catenin pathway etc., but we are far from an overall picture [2, 17]. Several differentially expressed microRNAs have been described among malignant and benign adrenocortical tumors and normal adrenocortical tissue. Here we discuss those significantly differentially expressed ACC-associated microRNAs in detail that have been validated in at least two independent studies (microRNAs highlighted in bold in Table 1).
Table 1.
Validated microRNAs significantly differentially expressed in studies involving ACC samples
| Study | Sample source and method of analysis | Sample distribution | Validated microRNAs in ACC relative to adenomas or normal adrenal cortices |
|---|---|---|---|
| Tömböl et al. [24] | Tissue—TLDA | 7 ACC, 19 ACA, 10 NA | miR-503↑, miR-184↑, miR-210↑, miR-214↓, miR-511↓, miR-375↓ |
| Soon et al. [19] | Tissue—microarray | 22 ACC, 27 ACA, 4 NA | miR-195↓*, miR-335↓, miR-483-5p↑* |
| Patterson et al. [18] | Tissue—microarray | 10 ACC, 26 ACA, 21 NA‡ | miR-100↓, miR-125b↓, miR-195↓, miR-483-5p↑, miR-483-3p↑ |
| Özata et al. [20] | Tissue—microarray | 25 ACC, 43 ACA, 10 NA | miR-483-5p↑, miR-483-3p↑, miR-210↑, miR-21↑, miR-1974↓, miR-195↓, miR-497↓, miR-503↑*, miR-1202↑*, miR-1275↑* |
| Schmitz et al. [29] | Tissue (FFPE)—TLDA | 4 ACC, 9 ACA, 4 NA + validation cohort (n = 15) | miR-139-3p↓, miR-675↓, miR-335↓ |
| Chabre et al. [31] | Tissue—microarray | 12 ACC†, 6 ACA | miR-195↓, miR-335↓, miR-483-5p↑, miR-139-5p↑**, miR-376a↑** |
| Serum—qRT-PCR | 23 ACC†, 14 ACA, 9 NA | miR-195↓, miR-335↓, miR-483-5p↑** | |
| Szabó et al. [62] | Plasma—qRT-PCR | 13 ACC, 12 ACA | miR-100↑, miR-181b↑, miR-184↑, miR-210↑, miR-483-5p↑ |
| Patel et al. [63] | Serum—qRT-PCR | 17 ACC, 22 ACA | miR-34a↑, miR-483-5p↑ |
microRNAs highlighted in bold have been validated in at least two independent studies
↓ down-regulation, ↑ up-regulation relative to adenoma or normal adrenal, ACC adrenocortical cancer, ACA adrenocortical adenoma, NA normal adrenal cortex, ‡ NA were pooled in this study, † ACC were subdivided in this study in aggressive and non-aggressive subgroups, * associated with shorter survival, ** Overexpressed in aggressive ACC relative to non-aggressive ACC, TLDA TaqMan low-density array, qRT-PCR quantitative real-time polymerase chain reaction
Relevance of microRNAs in ACC pathogenesis: differentially expressed microRNAs and affected pathways
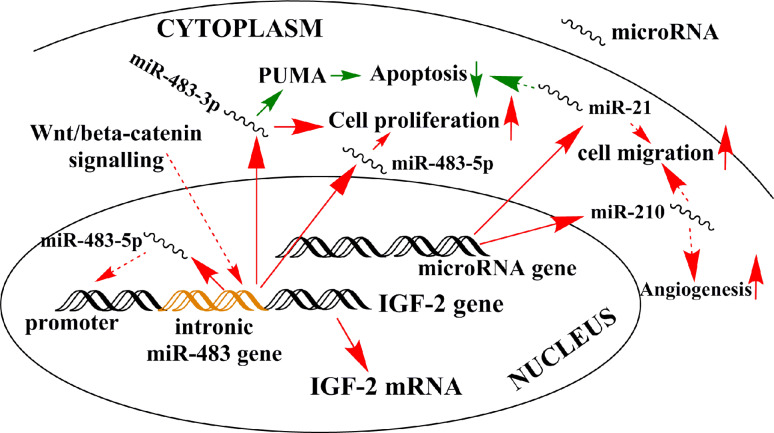
Among the overexpressed, oncogenic microRNAs in ACC, miR-483-5p and miR-483-3p, representing the two arms of the miR precursor transcribed from the miR-483 gene, are among the most consequently overexpressed microRNAs [18–20]. Its pathogenic role in ACC is supported by findings showing that inhibition of both miR-483-3p and miR-483-5p in vitro resulted in decreased cell proliferation, but only decreased miR-483-3p was associated with increased apoptosis [20]. The miR-483 gene is located within the second intron of the IGF2 gene, which is one of the most frequently overexpressed genes in ACC [17]. Furthermore, nuclear miR-483-5p was shown to enhance IGF-2 transcription via a hypothesized positive feedback loop in a Ewing sarcoma cell line [21], and if such a mechanism would exist in ACC, this would add a further layer of complexity in miR-483 action. miR-483-5p is activated by the Wnt/β-catenin pathway in lung adenocarcinoma [22], and affects cyclin–dependent kinase expression [23], but these interactions have not been demonstrated in ACC, yet. Overexpressed miR-483 can thus be hypothesized to be a major factor in ACC pathogenesis (Fig. 1 and Table 2).
Fig. 1.
Schematic representation of the potential relevance of overexpressed microRNAs in ACC pathogenesis. Continuous arrows represent validated interactions in ACC, whereas dashed arrows have been demonstrated in other tissues, but not yet in ACC. Red up-regulation, green down-regulation. In other tissues, nuclear miR-483-5p is involved in a positive regulatory loop with IGF-2, and Wnt/β-catenin signaling stimulates miR-483 transcription. Cytoplasmic miR-483-3p and miR-483-5p have been demonstrated to interact with different pathways. Although numerous data support the relevance of miR-21 and miR-210 in other tumors, the effects of these microRNAs have not been validated in ACC, yet
Table 2.
Validated mRNA targets and affected pathways of microRNAs relevant in ACC
| microRNA | Over- or underexpressed in ACC | Validated targets in the adrenal | Pathways affected | Reference | Validated targets in other tissues | Pathways affected | Reference(s) |
|---|---|---|---|---|---|---|---|
| miR-483-3p | Overexpressed | PUMA | Apoptosis | [20] | CDC25A | Cell cycle | [23] |
| miR-483-5p | Overexpressed |
RhoGDI1, ALCAM IGF-2 |
Cell migration, invasion growth factor |
[24] [22] |
|||
| miR-503 | Overexpressed | Phosphatidyl-inositol 3-kinase, inhibitor of κB kinase β, cyclin D1 |
Signaling Signaling cell cycle |
[25] [26] |
|||
| miR-195 | Underexpressed | DICER, TARBP2 | microRNA processing | [41] |
Cyclin D1, cyclin E1, CDC42, Stim 1, VEGF |
Cell cycle Cell cycle Cell migration Angiogenesis |
[33] [34] [35] [36] |
| miR-497 | Underexpressed | DICER, TARBP2 | microRNA processing | [41] | Checkpoint kinase 1, cyclin E1 |
Cell cycle Cell cycle |
[39] [40] |
| miR-335 | Underexpressed |
Formin family of actin nucleators BCL2L2 |
Cell migration, invasion Apoptosis |
[44] [47] |
|||
| miR-99a* | Underexpressed | IGFR1, mTOR | Signaling | [48] | IGFR1, mTOR | Signaling | [49] |
| miR-100* | Underexpressed | IGFR1, mTOR | Signaling | [48] |
PLK1 FKBP51 |
Cell cycle Signaling |
[50] [49] |
ALCAM activated leukocyte cell adhesion molecule, BCL2L2 B cell CLL/lymphoma 2 like 2, PUMA p53 upregulated modulator of apoptosis, CDC25a cell division cycle 25a, CDC42 cell division cycle 42, PLK1 polo-like kinase 1, RhoGDI1 rho GDP dissociation inhibitor alpha, Stim1 stromal interaction molecule 1, VEGF vascular endothelial growth factor, *validated in pediatric adrenal tumors
Overexpressed miR-503 has been validated in ACC [20, 24]. The molecular pathways affected by miR-503 in ACC are unknown, but mRNAs of proteins involved in cell signaling and the cell cycle have been validated in other tumors [25, 26] (Table 2).
miR-210 overexpression has been described in a wide range of different malignancies including ACC [20, 24]. miR-210 is induced by hypoxia inducible factor 1α (HIF1α) and is the major hypoxia-associated microRNA overexpressed under hypoxic conditions often encountered in malignant neoplasms [27]. miR-210 is involved in the regulation of angiogenesis, mitochondrial energy metabolism, DNA repair, apoptosis and the cell cycle via affecting a wide range of mRNAs [27]. In a most recent study, miR-210 overexpression has been associated with aggressive ACC behavior characterized by necrosis and high Ki-67 proliferative index [28].
Overexpressed miR-675 is interesting [29], as its gene is embedded in the first exon of the long non-coding RNA H19 [28] forming part of the imprinted IGF2/H19 locus [17]. The receptor for IGF2, insulin-like growth factor receptor 1 is a potential target of miR-675 [30]. Since H19 is usually underexpressed in ACC [31], the overexpression of miR-675 is difficult to explain.
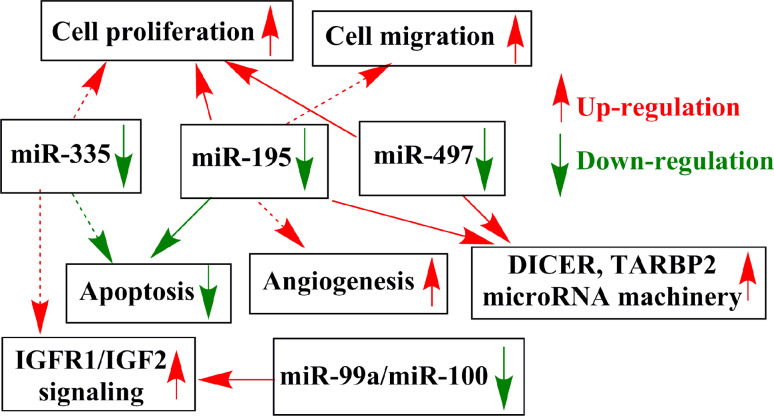
Several underexpressed microRNAs have been validated in ACC. Down-regulation of the tumor suppressor let-7 microRNA family member miR-195 has been described in several studies [18–20, 32] and overexpressed miR-195 inhibited ACC proliferation in vitro [20]. miR-195 affects pathways involved in the cell cycle, cell migration, signaling, and angiogenesis [33–36]. Vascular endothelial growth factor (VEGF) is among its validated targets in hepatocellular carcinoma [35], and underexpressed miR-195 might be involved in the overexpression of VEGF characteristic for ACC [37]. By our in silico gene expression network analysis, underexpressed miR-195 forms part of the core layer of the regulatory network as a major hub interacting with several other genes including the c-MYC oncogene [38]. Another let-7 family member, miR-497, has also been found to be underexpressed in ACC, and its overexpression in vitro has lead to decreased cell proliferation in adrenocortical cells [20] and in other tissues it was shown to affect cell cycle regulation [39, 40] (Fig. 2 and Table 2).
Fig. 2.
Schematic representation of the potential relevance of underexpressed microRNAs in ACC pathogenesis. Continuous arrows represent validated interactions in ACC, whereas dashed arrows have been demonstrated in other tissues, but not yet in ACC. Red up-regulation, green down-regulation
It is interesting to note that three microRNA-processing enzymes, i.e., DICER, TARBP2, and DROSHA, are overexpressed in ACC compared to adrenocortical adenoma (ACA), and among these, DICER and TARBP2 appear to be targets of both miR-195 and miR-497 [41]. This observation highlights the remarkable interplay between microRNAs and the microRNA-processing machinery, whereby microRNAs themselves might be implicated in the dysregulation of microRNA expression in ACC.
miR-335 underexpression has been validated in three studies [19, 29, 32] and this microRNA is considered to be a potent tumor suppressor microRNA in several human cancer tissues [42, 43]. No data are available yet on its targets in the adrenal cortex, but in other tissues miR-335 suppresses tumor cell migration, invasion, and metastasis [44–47] (Table 2).
In the only study involving pediatric adrenal tumors, miR-99a and miR-100 were identified as the most significantly down-regulated microRNAs. IGFR1 and mTOR (mammalian target of rapamycin)/raptor mRNAs were established as targets of these microRNAs in both adrenal and other tissues [48, 49] along with targets involved in cell cycle regulation [50] (Table 2).
From the pool of several other differentially expressed microRNAs described in adrenocortical tumors, but unconfirmed in two independent studies, underexpressed miR-125b [18] and overexpressed miR-21 [20] are worth mentioning. miR-125b behaves as a tumor suppressor in several malignancies often accompanying miR-100 that can be related to their close chromosomal localization (11q) [51]. miR-21 is a widely overexpressed oncogenic microRNA [52] that affects many pathways involved in the regulation of the cell cycle, receptor signaling, apoptosis, etc. [53–55] (Table 2).
By analyzing the published microRNA profiling studies, we have found 39 microRNAs that are commonly altered in at least two reports [56]. The pathways affected by differentially expressed microRNAs involved the cell cycle, retinoic acid signaling (including aryl hydrocarbon and integrin signaling), and several other pathways whose biological relevance in ACC is unclear [56]. Altered expression of mRNAs coding for proteins regulating the G2-M cell cycle checkpoint has been established as the major microRNA-mediated pathway in our microRNA profiling study by bioinformatics tools [24]. In line with these data, cell-cycle checkpoint regulation and retinoic acid signaling have been identified as major pathogenic pathways in ACC by our meta-analysis of transcriptome datasets [57].
In a most recent study, next-generation sequencing (NGS) has been performed in a cohort of 45 ACC samples [58]. By the analysis of microRNA expression, three major tumor clusters could be established that could be correlated with the clinical behavior of ACC along with the other molecular alterations. Apart from overexpressed miR-483-5p, novel microRNAs including the up-regulated miR-506-514 cluster have been found [58]. This is the only study using the NGS approach to date for microRNA profiling in ACC, and the validation of the significantly differentially expressed microRNAs should be performed by another technique (like real-time PCR). Nevertheless, the molecular subclassification of ACC based on bioinformatics approaches is a major finding from a clinical point of view, as it could be used to select patients with the risk of recurrence and rapid progression for whom aggressive treatment strategy is warranted.
Despite several commonly altered microRNAs in different studies, an overall picture of microRNA expression changes in ACC is lacking. In order to obtain more homogeneous and conclusive data, international collaborative studies would be helpful to enable profiling on large sample sets on uniform methodological platforms (e.g., microarray or NGS validated by PCR-based methods) using uniform statistical analysis.
MicroRNAs in the diagnosis of adrenocortical cancer
Intensive research efforts are being conducted to find adrenocortical microRNA markers of malignancy and prognosis. The histological diagnosis of adrenocortical tumors is difficult, and the mostly used Weiss-scoring has severe limitations [2]. It is very difficult to exclude malignancy in large (>6 cm diameter) tumors and to establish malignancy in small (<4 cm diameter) tumors.
Overexpressed miR-483-5p [18, 19], underexpressed miR-195 [19], the expressional difference of miR-503 and miR-511 [24], and overexpressed miR-335 and miR-675 [29] have been proposed as reliable markers of malignancy. Overexpressed miR-483-5p [19], miR-503, miR-1202, and miR-1275 [20] and underexpressed miR-195 [19] have been associated with poor survival in ACC. Despite the high sensitivity and specificity values reported for some of these microRNAs (e.g., 100 % positive predictive and 92 % negative predictive value for miR-483-5p [18] and 100 % sensitivity and 97 % specificity for miR-511-miR-503 [24]), the patient cohorts were mostly small and therefore larger-scale studies are warranted to confirm the clinical utility of these tissue microRNAs. Moreover, the hormonal features of tumors have been taken into account in two studies [24, 29], whereas others have included samples irrespective of their hormonal activity [18–20]. Since microRNA patterns can be associated with differences in hormone secretion [59], such discrepancies in the composition of patient cohorts might be relevant [60]. For the better evaluation of the diagnostic or prognostic role of certain microRNAs in adrenocortical tumors, the direct assessment of microRNAs in adrenal tissues by in situ hybridization would be a novel possibility [61].
Circulating microRNAs might be exploited as minimally invasive markers of malignancy enabling an early preoperative diagnosis [9]. Three studies have been published to date reporting on the analysis of circulating microRNAs in adrenocortical tumors. Overexpression of miR-483-5p paralleling observations made in ACC tissues has been confirmed in all studies [31, 62, 63]. In the study by Chabre et al., overexpressed miR-483-5p and underexpressed miR-195 could be exploited for the differentiation for aggressive and non-aggressive ACC [31] that could be of major relevance for treatment planning. Overexpression of miR-100, miR-181b, miR-184, and miR-210 in ACC plasma samples have been noted in our study [62], whereas overexpressed miR-34a has been described in Patel’s study [63]. The overexpression of serum miR-34a is difficult to explain as it is mostly regarded as a tumor suppressor and is underexpressed in several malignancies [64] including ACC by microarray [18]. The sensitivity and specificity values reported for these blood-borne microRNAs do not appear to be high enough for clinical introduction as malignancy markers at present [62, 63].
There are several technical difficulties associated with the analysis of circulating microRNAs [62, 65, 66]. The low RNA yield of blood samples and the lack of reliable methods for quantity assessment of blood-borne microRNAs makes the standardization of data difficult. The profiling method is also a matter of debate, since microarray, PCR, and most recently NGS have been proposed. We suggest the use of PCR for circulating microRNA profiling [62], but NGS might also be promising in the future. The method of raw data normalization, the choice of reference gene is another major issue. Synthetic spike-in-RNAs (e.g., cel-miR-39) has been used as reference in several studies including ours [62], but these cannot be regarded as biological controls. Circulating microRNAs with relatively stable expression like miR-16 was useful in some studies [62, 65], and the combination of different reference genes can also be proposed [62].
MicroRNAs in pheochromocytomas
MicroRNA expression profiling in pheochromocytomas (PCC) and paragangliomas (PGL, extraadrenal pheochromocytoma) has been reported in four studies to date. It was found that 25–30 % of PCC arise in the context of hereditary tumor syndromes including multiple endocrine neoplasia type 2 (MEN2), von Hippel-Lindau disease (VHL), neurofibromatosis type 1 (NF1), hereditary paraganglioma syndromes caused by germline mutations of RET, VHL, NF1, SDHD, SDHAF2, SDHC, SDHB and SDHA (SDH: succinate dehydrogenase) genes, respectively [67]. Recently, mutations of two novel genes, TMEM127 and MAX have been found to be associated with PCC as well [68, 69]. The molecular pathogenesis of PCC follows two main routes: (i) pseudohypoxia-neoangiogenesis pathways linked to the overactivation HIF1α (mutations in SDH and VHL genes, cluster 1), (ii) activation of PI3K/AKT/mTOR/Ras signaling characteristic for RET, NF1, TMEM127, and MAX mutations (cluster 2) [67]. These two clusters are also clearly distinct in their transcriptional (mRNA) profiles [69]. PCCs are mostly benign, but their benign or malignant behavior cannot be predicted by histological analysis [3, 4]. Malignancy is frequent in SDHB-associated PCC. There are no reliable markers of malignancy or recurrence [3, 4], and microRNAs might be promising in this respect.
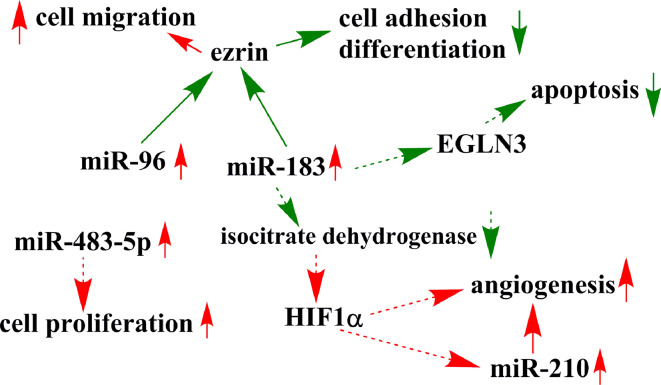
MicroRNA profiling has been performed in hereditary tumor syndrome-associated PCC (Table 3). MicroRNAs as genetic group markers have been established characteristic for clusters 1 and 2 similar to the clustering based on the transcriptome. Overexpression of hypoxia-related miR-210 was found in pseudohypoxia-associated highly vascularized SDHB- and VHL-associated tumors [71]. Overexpression of miR-139-3p, miR-541, miR-765, and miR-133b have been found characteristic for VHL tumors [71, 72]. Overexpressed miR-96 [71] and miR-183 [71, 73] was described in SDHB-associated pheochromocytomas, and the transfection of these two microRNAs hampered the nerve growth factor (NGF)-induced differentiation of rat PC12 cells in vitro [70]. NGF treatment of PC12 resulted in the significant underexpression of miR-139-3p and miR-210, which are overexpressed in VHL- and SDHB + VHL-tumors, respectively [74]. Furthermore, the cytoskeleton-associated protein ezrin, involved in cell adhesion, migration, and neuronal differentiation, was validated as a target of miR-96 and miR-183 [71]. miR-96 and miR-183 belong to the “miR-183 family” of oncogenic microRNAs that are overexpressed in a wide array of human cancers [75–77] (Table 4). Overexpressed miR-183 was hypothesized to interfere with EGLN3 (EGL-nine) expression involved in the regulation of neuronal apoptosis [71]. Moreover, miR-183 has been shown to up-regulate HIF1α via targeting isocitrate dehydrogenase 2 in glioma [78]. If a similar interaction could be demonstrated in SDHB-associated PCC, this might be implicated in the pseudohypoxia phenotype (Fig. 3). In our meta-analysis on neural crest tumors (PCC and neuroblastoma), we have noted the overexpression of EGLN mRNA in PCC that could be correlated with down-regulated miR-132 in VHL-associated tumors [79].
Table 3.
Validated microRNAs significantly differentially expressed in pheochromocytomas reported in four studies
| General PCC | MEN2 | VHL | SDHB | SDHD | MAX | Recurring PCC | Malignant PCC | |
|---|---|---|---|---|---|---|---|---|
| de Cubas et al. [71] | miR-137↑* |
miR-382↑ miR-488↑ miR-885-5p↑ |
miR-382↑ miR-210↑ miR-133b↑ |
miR-382↑ miR-210↑ miR-183↑ miR-96↑ |
miR-382↑ | miR-382↓ | ||
| Patterson et al. [73] |
miR-483-5p↑ miR-101↑ miR-183↑ |
miR-483-5p↑** miR-101↑ miR-183↑ |
||||||
| Tömböl et al. [72] | miR-885-5p ↑ |
miR-139-3p↑ miR-541↑ miR-765↑ |
miR-1225-3p↑ | |||||
| Meyer-Rochow et al. [87] |
miR-483-5p ↑ miR-15a↓ miR-16↓ |
microRNAs in bold have been described in two studies
↓ down-regulation, ↑ up-regulation relative to sporadic PCC, * except for MAX-related PCC, ** Patterson et al. studied only SDHB-associated malignant PCC
Table 4.
Validated mRNA targets of microRNAs relevant in pheochromocytoma
| microRNA | Validated targets in the adrenal | Principal function of target mRNA | Reference | Validated targets in other tissues | Principal function of target mRNA | Reference |
|---|---|---|---|---|---|---|
| miR-96 | Ezrin | Cell adhesion, migration | [70] | FOXO1 | Transcription factor | [75, 76] |
| miR-183 | Ezrin | Cell adhesion, migration | [70] |
SMAD4 Isocitrate dehydrogenase 2 |
Signaling, apoptosis Energy metabolism |
[77] [78] |
| miR-885-5p |
Caspase 3, CDK2, MCM5 |
Apoptosis Cell cycle |
[80] [81] |
|||
| miR-137 |
KDM1A, Ezh2 histone methyltransferase, neurofibromin 1 |
Gene expression regulation, Cell signaling |
[82] [83] [84] |
|||
| miR-382 | c-MYC | Cell cycle, apoptosis | [85] | |||
| miR-15a |
BCL2, MCL1 Cyclin D1 |
Apoptosis Cell cycle |
[88] | |||
| miR-16 |
BLC2, MCL1 Cyclin D1 |
Apoptosis Cell cycle |
[88] |
BCL2 B-cell CLL/lymphoma 2, CDK2 cyclin-dependent kinase 2, KDM1A histone demethylase, lysine-specific demethylase 1, MCL1 myeloid cell leukemia 1, MCM5 mini-chromosome maintenance complex component 5
Fig. 3.
Schematic representation of the potential relevance of microRNAs in SDHB-mutation-associated pheochromocytoma. Continuous arrows represent validated interactions in PCC, whereas dashed arrows have been demonstrated in other tissues, but not yet in PCC. The interaction between miR-183 and EGLN3 has been hypothesized in PCC, but it has not yet been validated. Red up-regulation, green down-regulation
Overexpression of miR-885-5p was observed in MEN2-associated PCC [71, 72] together with miR-488 [71]. miR-885-5p appears to target mRNAs of proteins involved in the regulation of apoptosis [80] and cell cycle [81] in other tissues (Table 4). By our in silico meta-analysis, the involvement of insulin-like growth factor 1 (IGF1) pathway in the pathogenesis of MEN2-associated PCC has been raised, and miR-885-5p might target IGF-binding proteins (IGFBP3, IGFBP7) [78].
Two microRNAs, miR-137 and miR-382, were overexpressed in most PCC, and therefore can be regarded as general PCC markers [72]. In MAX-mutation-related PCC, however, these two microRNAs were underexpressed, thus MAX-mutations might result in a microRNA profile distinct from other PCC classes. miR-137 is underexpressed in several malignancies and its targets include mRNAs involved in gene expression regulation and signaling [82–84]. The oncogene c-MYC was found among the validated targets of miR-382 [85] that might be relevant as MAX (MYC-associated factor X) forms part of the c-MYC network [69].
We have observed the overexpression of miR-1225-3p in recurring PCC compared to sporadic non-recurring, MEN2-, VHL-, and NF1-associated PCC. By pathway analysis, overexpressed miR-1225-3p might result in decreased Notch signaling [72]. The relevance of Notch signaling is underlined by in vitro studies on rat PC12 PCC cells where histone deacetylase inhibitors up-regulating Notch-1 inhibited cell proliferation [86]. Notch signaling might thus represent a novel target of PCC treatment.
The microRNA expression in malignant PCC has been investigated in two studies [73, 87] (Table 3). Overexpressed miR-483-5p was described in both studies. miR-483-5p is thus overexpressed in both malignant adrenocortical and adrenomedullary tumors despite their different embryogenic origin (cortex is mesodermal, medulla is ectodermal). Underexpressed miR-15a and miR-16 described by Meyer-Rochow et al. in malignant PCC [87] can be regarded as general tumor suppressor microRNAs down-regulated in several neoplasms targeting mRNAs of proteins involved in apoptosis and cell cycle regulation [88]. Transfection of miR-15a and miR-16 to rat PC12 cells inhibited their growth supporting their tumor suppressing activity in PCC, as well [87]. Three circulating microRNAs (miR-101, miR-183, and miR-483-5p) have been investigated as markers of PCC malignancy, but no significant differences in expression were found [73].
Perspective for microRNAs as direct or indirect targets for treatment
Being involved in basic cell biological processes leading to cancer development, microRNAs can be regarded as potential and potent targets for anti-cancer therapy. MicroRNAs could represent both direct and indirect targets. Here we mean direct targeting if the microRNA is directly affected by molecular approaches (antagonizing oncogenic miR by e.g., antagomirs, or increasing endogenous tumor suppressor microRNA expression by microRNA mimics, etc.) [14], whereas by indirect targeting the pathways established by microRNA profiling studies could be affected.
MicroRNAs as direct targets
Given their tissue-specific way of action, selecting microRNAs as treatment targets is difficult. Those microRNAs could be preferred, the expression of which is changed in the same direction in a wide array of malignancies. MicroRNAs have been proposed to overcome tumor chemo- and radiation resistance [5, 89]. We would propose those microRNAs for consideration whose targets and affected pathways have already been described and validated in several different tumor models. From the pool of relevant microRNAs in ACC and validated in at least two independent studies, miR-483-5p/miR-483-3p, miR-195, and miR-210 would be the most suitable candidates.
There are some examples on the potential anti-tumor applicability of these microRNAs in other tumors. In breast cancer cells, up-regulation of miR-195 expression increased their sensitivity to adriamycin treatment [90]. Knockdown of miR-210 facilitated radiation therapy in a human hepatoma xenograft model [91].
There are several major difficulties associated to the modulation of endogenous microRNA expression. Since the major goal of microRNA modulation would be most valuable in advanced tumor patients, systemic therapy would be envisaged. Apart from technical issues such as problems of administration, major unforeseen side effects could develop in part due to potential off-target actions of microRNAs related to the tissue-specific nature of microRNA action [14].
Indirect targets: pathways affected by microRNAs
The pathways established by microRNA profiling studies could also serve as therapeutical targets. The mTOR/Raptor signaling affected by miR99a/miR100 in childhood ACC could be influenced by the mTOR inhibitor everolimus demonstrated by in vitro and xenograft observations [48]. Despite these promising experimental data, however, everolimus does not appear to be an efficient therapeutic modality for ACC in the clinical setting [92]. Notch-signaling might be another example established in our study on recurring PCC [72] that could be exploited in the treatment by e.g., histone deacetylase inhibitors [86].
Among the other potential pathways that can be suggested by microRNA studies, retinoic acid signaling in ACC [56] might be of interest, and our in vitro and xenograft studies support the potential applicability of 9-cis-retinoic acid in ACC treatment [93]. Other, but currently unvalidated pathways such as aryl hydrocarbon and integrin signaling [56] might also include targets that could be exploited in therapy.
Studies on gene expression networks might be helpful for identifying the most relevant targets [38].
Since both ACC and malignant PCC are rare tumors, rapid advances in the clinical introduction of microRNA modulating approaches are hardly expected.
Conclusions
Altered expression of microRNAs has been described in several studies both in adrenocortical and adrenomedullary tumors. Their implication in adrenal tumorigenesis appears to be relevant, and their diagnostic utility is promising both as tissue and circulating biomarkers. There are, however, major discrepancies in the results from different research groups that can be associated with the different platforms, methodological approaches, patient cohorts, etc. Extensive experimental work-up and validation on larger patient cohorts will be needed to define the microRNA sets that can be reliably used for diagnosis. There is great potential for microRNAs in cancer treatment, but the clinical applicability of direct microRNA targeting for adrenal tumors seems to be, however, quite far away.
Acknowledgments
This study has been supported by the Hungarian Scientific Research Fund (OTKA K100295).
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Arnaldi G, Boscaro M. Adrenal incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26(4):405–419. doi: 10.1016/j.beem.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98(12):4551–4564. doi: 10.1210/jc.2013-3020. [DOI] [PubMed] [Google Scholar]
- 3.Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbeck’s Arch Surg. 2012;397(2):155–177. doi: 10.1007/s00423-011-0880-x. [DOI] [PubMed] [Google Scholar]
- 4.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14(3):569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 5.Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–1133. doi: 10.1093/carcin/bgs140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malumbres M. miRNAs and cancer: an epigenetics view. Mol Asp Med. 2013;34(4):863–874. doi: 10.1016/j.mam.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132(21):4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 8.Salmanidis M, Pillman K, Goodall G, Bracken C. Direct transcriptional regulation by nuclear microRNAs. Int J Biochem Cell Biol. 2014 doi: 10.1016/j.biocel.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. New Engl J Med. 2005;353(17):1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 10.He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102(52):19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klopfleisch R, Weiss AT, Gruber AD. Excavation of a buried treasure–DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol Histopathol. 2011;26(6):797–810. doi: 10.14670/HH-26.797. [DOI] [PubMed] [Google Scholar]
- 12.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids–the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8(8):467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11(3):145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 14.McDermott AM, Heneghan HM, Miller N, Kerin MJ. The therapeutic potential of microRNAs: disease modulators and drug targets. Pharm Res. 2011;28(12):3016–3029. doi: 10.1007/s11095-011-0550-2. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie W, Rasko JE, Flamant S. MicroRNA target prediction and validation. Adv Exp Med Biol. 2013;774:39–53. doi: 10.1007/978-94-007-5590-1_3. [DOI] [PubMed] [Google Scholar]
- 16.Page GP, Zakharkin SO, Kim K, Mehta T, Chen L, Zhang K. Microarray analysis. Methods Mol Biol. 2007;404:409–430. doi: 10.1007/978-1-59745-530-5_20. [DOI] [PubMed] [Google Scholar]
- 17.Bertherat J, Bertagna X. Pathogenesis of adrenocortical cancer. Best Pract Res Clin Endocrinol Metab. 2009;23(2):261–271. doi: 10.1016/j.beem.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Patterson EE, Holloway AK, Weng J, Fojo T, Kebebew E. MicroRNA profiling of adrenocortical tumors reveals miR-483 as a marker of malignancy. Cancer. 2011;117(8):1630–1639. doi: 10.1002/cncr.25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soon PS, Tacon LJ, Gill AJ, Bambach CP, Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson BG, Sidhu SB. miR-195 and miR-483-5p identified as predictors of poor prognosis in adrenocortical cancer. Clin Cancer Res. 2009;15(24):7684–7692. doi: 10.1158/1078-0432.CCR-09-1587. [DOI] [PubMed] [Google Scholar]
- 20.Özata DM, Caramuta S, Velazquez-Fernandez D, Akcakaya P, Xie H, Hoog A, Zedenius J, Backdahl M, Larsson C, Lui WO. The role of microRNA deregulation in the pathogenesis of adrenocortical carcinoma. Endocr-Relat Cancer. 2011;18(6):643–655. doi: 10.1530/ERC-11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Roth A, Yu M, Morris R, Bersani F, Rivera MN, Lu J, Shioda T, Vasudevan S, Ramaswamy S, Maheswaran S, Diederichs S, Haber DA. The IGF2 intronic miR-483 selectively enhances transcription from IGF2 fetal promoters and enhances tumorigenesis. Genes Dev. 2013;27(23):2543–2548. doi: 10.1101/gad.224170.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Q, Xu Y, Yang C, Chen Z, Jia C, Chen J, Zhang Y, Lai P, Fan X, Zhou X, Lin J, Li M, Ma W, Luo S, Bai X. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74(11):3031–3042. doi: 10.1158/0008-5472.CAN-13-2193. [DOI] [PubMed] [Google Scholar]
- 23.Bertero T, Bourget-Ponzio I, Puissant A, Loubat A, Mari B, Meneguzzi G, Auberger P, Barbry P, Ponzio G, Rezzonico R. Tumor suppressor function of miR-483-3p on squamous cell carcinomas due to its pro-apoptotic properties. Cell Cycle. 2013;12(14):2183–2193. doi: 10.4161/cc.25330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tömböl Z, Szabó PM, Molnar V, Wiener Z, Tolgyesi G, Horanyi J, Riesz P, Reismann P, Patocs A, Liko I, Gaillard RC, Falus A, Racz K, Igaz P. Integrative molecular bioinformatics study of human adrenocortical tumors: microRNA, tissue-specific target prediction, and pathway analysis. Endocr Relat Cancer. 2009;16(3):895–906. doi: 10.1677/ERC-09-0096. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Liu L, Zhang Y, Guan H, Wu J, Zhu X, Yuan J, Li M. MiR-503 targets PI3K p85 and IKK-beta and suppresses progression of non-small cell lung cancer. Int J Cancer. 2014;135(7):1531–1542. doi: 10.1002/ijc.28799. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Q, Feng MG, Mo YY. Systematic validation of predicted microRNAs for cyclin D1. BMC Cancer. 2009;9:194. doi: 10.1186/1471-2407-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin C, Greco S, Martelli F, Ivan M. miR-210: more than a silent player in hypoxia. IUBMB Life. 2011;63(2):94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duregon E, Rapa I, Votta A, Giorcelli J, Daffara F, Terzolo M, Scagliotti GV, Volante M, Papotti M. MicroRNA expression patterns in adrenocortical carcinoma variants and clinical pathologic correlations. Hum Pathol. 2014 doi: 10.1016/j.humpath.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KJ, Helwig J, Bertram S, Sheu SY, Suttorp AC, Seggewiss J, Willscher E, Walz MK, Worm K, Schmid KW. Differential expression of microRNA-675, microRNA-139-3p and microRNA-335 in benign and malignant adrenocortical tumors. J Clin Pathol. 2011;64(6):529–535. doi: 10.1136/jcp.2010.085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G, Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14(7):659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribeiro TC, Latronico AC. Insulin-like growth factor system on adrenocortical tumorigenesis. Mol Cell Endocrinol. 2012;351(1):96–100. doi: 10.1016/j.mce.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 32.Chabre O, Libe R, Assie G, Barreau O, Bertherat J, Bertagna X, Feige JJ, Cherradi N. Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr Relat Cancer. 2013;20(4):579–594. doi: 10.1530/ERC-13-0051. [DOI] [PubMed] [Google Scholar]
- 33.Hui W, Yuntao L, Lun L, WenSheng L, ChaoFeng L, HaiYong H, Yueyang B. MicroRNA-195 inhibits the proliferation of human glioma cells by directly targeting cyclin D1 and cyclin E1. PLoS ONE. 2013;8(1):e54932. doi: 10.1371/journal.pone.0054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhuang R, Rao JN, Zou T, Liu L, Xiao L, Cao S, Hansraj NZ, Gorospe M, Wang JY. miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration. Nucleic Acids Resh. 2013;41(16):7905–7919. doi: 10.1093/nar/gkt565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu MG, Li S, Yu TT, Qian LJ, Cao RS, Zhu H, Xiao B, Jiao CH, Tang NN, Ma JJ, Hua J, Zhang WF, Zhang HJ, Shi RH. Differential expression of miR-195 in esophageal squamous cell carcinoma and miR-195 expression inhibits tumor cell proliferation and invasion by targeting of Cdc42. FEBS Lett. 2013;587(21):3471–3479. doi: 10.1016/j.febslet.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Zhao N, Li S, Fang JH, Chen MX, Yang J, Jia WH, Yuan Y, Zhuang SM. MicroRNA-195 suppresses angiogenesis and metastasis of hepatocellular carcinoma by inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology. 2013;58(2):642–653. doi: 10.1002/hep.26373. [DOI] [PubMed] [Google Scholar]
- 37.Bernini GP, Moretti A, Bonadio AG, Menicagli M, Viacava P, Naccarato AG, Iacconi P, Miccoli P, Salvetti A. Angiogenesis in human normal and pathologic adrenal cortex. J Clin Endocrinol Metab. 2002;87(11):4961–4965. doi: 10.1210/jc.2001-011799. [DOI] [PubMed] [Google Scholar]
- 38.Szabó PM, Butz H, Igaz P, Racz K, Hunyady L, Patocs A. Minireview: miRomics in endocrinology: a novel approach for modeling endocrine diseases. Mol Endocrinol. 2013;27(4):573–585. doi: 10.1210/me.2012-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF, Wang HY. Checkpoint kinase 1 is negatively regulated by miR-497 in hepatocellular carcinoma. Med Oncol. 2014;31(3):844. doi: 10.1007/s12032-014-0844-4. [DOI] [PubMed] [Google Scholar]
- 40.Luo Q, Li X, Gao Y, Long Y, Chen L, Huang Y, Fang L. MiRNA-497 regulates cell growth and invasion by targeting cyclin E1 in breast cancer. Cancer Cell Int. 2013;13(1):95. doi: 10.1186/1475-2867-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caramuta S, Lee L, Ozata DM, Akcakaya P, Xie H, Hoog A, Zedenius J, Backdahl M, Larsson C, Lui WO. Clinical and functional impact of TARBP2 over-expression in adrenocortical carcinoma. Endocr Relat Cancer. 2013;20(4):551–564. doi: 10.1530/ERC-13-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao J, Cai J, Huang D, Han Q, Chen Y, Yang Q, Yang C, Kuang Y, Li D, Wang Z. miR-335 represents an independent prognostic marker in epithelial ovarian cancer. Am J Clin Pathol. 2014;141(3):437–442. doi: 10.1309/AJCPLYTZGB54ISZC. [DOI] [PubMed] [Google Scholar]
- 43.Xiong SW, Lin TX, Xu KW, Dong W, Ling XH, Jiang FN, Chen G, Zhong WD, Huang J. MicroRNA-335 acts as a candidate tumor suppressor in prostate cancer. Pathol Oncol Res. 2013;19(3):529–537. doi: 10.1007/s12253-013-9613-5. [DOI] [PubMed] [Google Scholar]
- 44.Lynch J, Meehan MH, Crean J, Copeland J, Stallings RL, Bray IM. Metastasis suppressor microRNA-335 targets the formin family of actin nucleators. PLoS One. 2013;8(11):e78428. doi: 10.1371/journal.pone.0078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Zhao W, Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol Cell Biochem. 2013;384(1–2):105–111. doi: 10.1007/s11010-013-1786-4. [DOI] [PubMed] [Google Scholar]
- 46.Gong M, Ma J, Guillemette R, Zhou M, Yang Y, Yang Y, Hock JM, Yu X. miR-335 inhibits small cell lung cancer bone metastases via IGF-IR and RANKL pathways. Mol Cancer Res. 2014;12(1):101–110. doi: 10.1158/1541-7786.MCR-13-0136. [DOI] [PubMed] [Google Scholar]
- 47.Cao J, Cai J, Huang D, Han Q, Yang Q, Li T, Ding H, Wang Z. miR-335 represents an invasion suppressor gene in ovarian cancer by targeting Bcl-w. Oncol Rep. 2013;30(2):701–706. doi: 10.3892/or.2013.2482. [DOI] [PubMed] [Google Scholar]
- 48.Doghman M, El Wakil A, Cardinaud B, Thomas E, Wang J, Zhao W, Peralta-Del Valle MH, Figueiredo BC, Zambetti GP, Lalli E. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70(11):4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li XJ, Luo XQ, Han BW, Duan FT, Wei PP, Chen YQ. MicroRNA-100/99a, deregulated in acute lymphoblastic leukaemia, suppress proliferation and promote apoptosis by regulating the FKBP51 and IGF1R/mTOR signalling pathways. Br J Cancer. 2013;109(8):2189–2198. doi: 10.1038/bjc.2013.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Lu KH, Liu ZL, Sun M, De W, Wang ZX. MicroRNA-100 is a potential molecular marker of non-small cell lung cancer and functions as a tumor suppressor by targeting polo-like kinase 1. BMC Cancer. 2012;12:519. doi: 10.1186/1471-2407-12-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Henson BJ, Bhattacharjee S, O’Dee DM, Feingold E, Gollin SM. Decreased expression of miR-125b and miR-100 in oral cancer cells contributes to malignancy. Genes Chromosomes Cancer. 2009;48(7):569–582. doi: 10.1002/gcc.20666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baer C, Claus R, Plass C. Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res. 2013;73(2):473–477. doi: 10.1158/0008-5472.CAN-12-3731. [DOI] [PubMed] [Google Scholar]
- 53.Gao W, Xu J, Liu L, Shen H, Zeng H, Shu Y. A systematic-analysis of predicted miR-21 targets identifies a signature for lung cancer. Biomed Pharmacother. 2012;66(1):21–28. doi: 10.1016/j.biopha.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8(5):706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong L, Han Y, Zhang Y, Zhang H, Zhao Q, Wu K, Fan D. MicroRNA-21: a therapeutic target for reversing drug resistance in cancer. Exp Opin Ther Targets. 2013;17(9):1073–1080. doi: 10.1517/14728222.2013.819853. [DOI] [PubMed] [Google Scholar]
- 56.Zsippai A, Szabó PM, Szabó DR, Nagy Z, Patocs A, Racz K, Igaz P. In silico analysis of pathways affected by differentially expressed microRNA in adrenocortical tumors. J Endocrinol Invest. 2013;36(11):1011–1019. doi: 10.3275/9024. [DOI] [PubMed] [Google Scholar]
- 57.Szabó PM, Tamasi V, Molnar V, Andrasfalvy M, Tömböl Z, Farkas R, Kovesdi K, Patocs A, Toth M, Szalai C, Falus A, Racz K, Igaz P. Meta-analysis of adrenocortical tumor genomics data: novel pathogenic pathways revealed. Oncogene. 2010;29(21):3163–3172. doi: 10.1038/onc.2010.80. [DOI] [PubMed] [Google Scholar]
- 58.Assie G, Letouze E, Fassnacht M, Jouinot A, Luscap W, Barreau O, Omeiri H, Rodriguez S, Perlemoine K, Rene-Corail F, Elarouci N, Sbiera S, Kroiss M, Allolio B, Waldmann J, Quinkler M, Mannelli M, Mantero F, Papathomas T, De Krijger R, Tabarin A, Kerlan V, Baudin E, Tissier F, Dousset B, Groussin L, Amar L, Clauser E, Bertagna X, Ragazzon B, Beuschlein F, Libe R, de Reynies A, Bertherat J. Integrated genomic characterization of adrenocortical carcinoma. Nat Gen. 2014;46(6):607–612. doi: 10.1038/ng.2953. [DOI] [PubMed] [Google Scholar]
- 59.Velazquez-Fernandez D, Caramuta S, Ozata DM, Lu M, Hoog A, Backdahl M, Larsson C, Lui WO, Zedenius J. MicroRNA expression patterns associated with hyperfunctioning and non-hyperfunctioning phenotypes in adrenocortical adenomas. Eur J Endocrinol. 2014;170(4):583–591. doi: 10.1530/EJE-13-0817. [DOI] [PubMed] [Google Scholar]
- 60.Tömböl Z, Szabó PM, Patocs A, Racz K, Igaz P. Differences in microRNA expression profiles of adrenocortical tumors–letter. Clin Cancer Res. 2010;16(10):2915. doi: 10.1158/1078-0432.CCR-10-0308. [DOI] [PubMed] [Google Scholar]
- 61.Hanna JA, Wimberly H, Kumar S, Slack F, Agarwal S, Rimm DL. Quantitative analysis of microRNAs in tissue microarrays by in situ hybridization. Biotechniques. 2012;52(4):235–245. doi: 10.2144/000113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szabó DR, Luconi M, Szabó PM, Toth M, Szucs N, Horanyi J, Nagy Z, Mannelli M, Patocs A, Racz K, Igaz P. Analysis of circulating microRNAs in adrenocortical tumors. Lab Invest. 2014;94(3):331–339. doi: 10.1038/labinvest.2013.148. [DOI] [PubMed] [Google Scholar]
- 63.Patel D, Boufraqech M, Jain M, Zhang L, He M, Gesuwan K, Gulati N, Nilubol N, Fojo T, Kebebew E. MiR-34a and miR-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery. 2013;154(6):1224–1228. doi: 10.1016/j.surg.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Zhou JY, Zhou JY. MicroRNA-34a: role in cancer and cardiovascular disease. Curr Drug Targets. 2014;15:361–373. doi: 10.2174/1389450115666140120102935. [DOI] [PubMed] [Google Scholar]
- 65.Brase JC, Wuttig D, Kuner R, Sültmann H. Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer. 2010;9:306. doi: 10.1186/1476-4598-9-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2011;80(2):193–208. doi: 10.1016/j.critrevonc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Favier J, Igaz P, Burnichon N, Amar L, Libe R, Badoual C, Tissier F, Bertherat J, Plouin PF, Jeunemaitre X, Gimenez-Roqueplo AP. Rationale for anti-angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23(1):34–42. doi: 10.1007/s12022-011-9189-0. [DOI] [PubMed] [Google Scholar]
- 68.Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, Lechleiter JD, Sass M, Aronin N, Schiavi F, Boaretto F, Opocher G, Toledo RA, Toledo SP, Stiles C, Aguiar RC, Dahia PL. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Gen. 2010;42(3):229–233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, Landa I, Leandro-Garcia LJ, Leton R, Honrado E, Ramos-Medina R, Caronia D, Pita G, Gomez-Grana A, de Cubas AA, Inglada-Perez L, Maliszewska A, Taschin E, Bobisse S, Pica G, Loli P, Hernandez-Lavado R, Diaz JA, Gomez-Morales M, Gonzalez-Neira A, Roncador G, Rodriguez-Antona C, Benitez J, Mannelli M, Opocher G, Robledo M, Cascon A. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Gen. 2011;43(7):663–667. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 70.Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res. 2012;44(5):328–333. doi: 10.1055/s-0031-1301302. [DOI] [PubMed] [Google Scholar]
- 71.de Cubas AA, Leandro-Garcia LJ, Schiavi F, Mancikova V, Comino-Mendez I, Inglada-Perez L, Perez-Martinez M, Ibarz N, Ximenez-Embun P, Lopez-Jimenez E, Maliszewska A, Leton R, Gomez Grana A, Bernal C, Alvarez-Escola C, Rodriguez-Antona C, Opocher G, Munoz J, Megias D, Cascon A, Robledo M. Integrative analysis of miRNA and mRNA expression profiles in pheochromocytoma and paraganglioma identifies genotype-specific markers and potentially regulated pathways. Endocr Relat Cancer. 2013;20(4):477–493. doi: 10.1530/ERC-12-0183. [DOI] [PubMed] [Google Scholar]
- 72.Tömböl Z, Eder K, Kovacs A, Szabó PM, Kulka J, Liko I, Zalatnai A, Racz G, Toth M, Patocs A, Falus A, Racz K, Igaz P. MicroRNA expression profiling in benign (sporadic and hereditary) and recurring adrenal pheochromocytomas. Mod Pathol. 2010;23(12):1583–1595. doi: 10.1038/modpathol.2010.164. [DOI] [PubMed] [Google Scholar]
- 73.Patterson E, Webb R, Weisbrod A, Bian B, He M, Zhang L, Holloway AK, Krishna R, Nilubol N, Pacak K, Kebebew E. The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endoc Relat Cancer. 2012;19(2):157–166. doi: 10.1530/ERC-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamada N, Fujita Y, Kojima T, Kitamoto A, Akao Y, Nozawa Y, Ito M. MicroRNA expression profiling of NGF-treated PC12 cells revealed a critical role for miR-221 in neuronal differentiation. Neurochem Int. 2012;60(8):743–750. doi: 10.1016/j.neuint.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 75.Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang Q, Cheng P, Tang ZH, Huang F. Meta-analysis of microRNA-183 family expression in human cancer studies comparing cancer tissues with noncancerous tissues. Gene. 2013;527(1):26–32. doi: 10.1016/j.gene.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Fendler A, Jung M, Stephan C, Erbersdobler A, Jung K, Yousef GM. The antiapoptotic function of miR-96 in prostate cancer by inhibition of FOXO1. PLoS One. 2013;8(11):e80807. doi: 10.1371/journal.pone.0080807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueno K, Hirata H, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Hinoda Y, Dahiya R. microRNA-183 is an oncogene targeting Dkk-3 and SMAD4 in prostate cancer. Br J Cancer. 2013;108(8):1659–1667. doi: 10.1038/bjc.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka H, Sasayama T, Tanaka K, Nakamizo S, Nishihara M, Mizukawa K, Kohta M, Koyama J, Miyake S, Taniguchi M, Hosoda K, Kohmura E. MicroRNA-183 upregulates HIF-1alpha by targeting isocitrate dehydrogenase 2 (IDH2) in glioma cells. J Neurooncol. 2013;111(3):273–283. doi: 10.1007/s11060-012-1027-9. [DOI] [PubMed] [Google Scholar]
- 79.Szabó PM, Pinter M, Szabó DR, Zsippai A, Patocs A, Falus A, Racz K, Igaz P. Integrative analysis of neuroblastoma and pheochromocytoma genomics data. BMC Med Genomics. 2012;5:48. doi: 10.1186/1755-8794-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guan X, Liu Z, Liu H, Yu H, Wang LE, Sturgis EM, Li G, Wei Q. A functional variant at the miR-885-5p binding site of CASP3 confers risk of both index and second primary malignancies in patients with head and neck cancer. FASEB J. 2013;27(4):1404–1412. doi: 10.1096/fj.12-223420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Afanasyeva EA, Mestdagh P, Kumps C, Vandesompele J, Ehemann V, Theissen J, Fischer M, Zapatka M, Brors B, Savelyeva L, Sagulenko V, Speleman F, Schwab M, Westermann F. MicroRNA miR-885-5p targets CDK2 and MCM5, activates p53 and inhibits proliferation and survival. Cell Death Differ. 2011;18(6):974–984. doi: 10.1038/cdd.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Althoff K, Beckers A, Odersky A, Mestdagh P, Koster J, Bray IM, Bryan K, Vandesompele J, Speleman F, Stallings RL, Schramm A, Eggert A, Sprussel A, Schulte JH. MiR-137 functions as a tumor suppressor in neuroblastoma by downregulating KDM1A. Int J Cancer. 2013;133(5):1064–1073. doi: 10.1002/ijc.28091. [DOI] [PubMed] [Google Scholar]
- 83.Szulwach KE, Li X, Smrt RD, Li Y, Luo Y, Lin L, Santistevan NJ, Li W, Zhao X, Jin P. Cross talk between microRNA and epigenetic regulation in adult neurogenesis. J Cell Biol. 2010;189(1):127–141. doi: 10.1083/jcb.200908151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paschou M, Doxakis E. Neurofibromin 1 is a miRNA target in neurons. PLoS One. 2012;7(10):e46773. doi: 10.1371/journal.pone.0046773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thayanithy V, Sarver AL, Kartha RV, Li L, Angstadt AY, Breen M, Steer CJ, Modiano JF, Subramanian S. Perturbation of 14q32 miRNAs-cMYC gene network in osteosarcoma. Bone. 2012;50(1):171–181. doi: 10.1016/j.bone.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adler JT, Hottinger DG, Kunnimalaiyaan M, Chen H. Histone deacetylase inhibitors upregulate Notch-1 and inhibit growth in pheochromocytoma cells. Surgery. 2008;144(6):956–961. doi: 10.1016/j.surg.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meyer-Rochow GY, Jackson NE, Conaglen JV, Whittle DE, Kunnimalaiyaan M, Chen H, Westin G, Sandgren J, Stalberg P, Khanafshar E, Shibru D, Duh QY, Clark OH, Kebebew E, Gill AJ, Clifton-Bligh R, Robinson BG, Benn DE, Sidhu SB. MicroRNA profiling of benign and malignant pheochromocytomas identifies novel diagnostic and therapeutic targets. Endocr Relat Cancer. 2010;17(3):835–846. doi: 10.1677/ERC-10-0142. [DOI] [PubMed] [Google Scholar]
- 88.Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010;17(2):215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Zhu H, Yang X, Ge Y, Zhang C, Qin Q, Lu J, Zhan L, Cheng H, Sun X. MicroRNA-21 is a novel promising target in cancer radiation therapy. Tumor Biol. 2014;35(5):3975–3979. doi: 10.1007/s13277-014-1623-8. [DOI] [PubMed] [Google Scholar]
- 90.Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, Weng Y. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013;30(2):877–889. doi: 10.3892/or.2013.2532. [DOI] [PubMed] [Google Scholar]
- 91.Yang W, Wei J, Sun T, Liu F. Effects of knockdown of miR-210 in combination with ionizing radiation on human hepatoma xenograft in nude mice. Rad Oncol. 2013;8:102. doi: 10.1186/1748-717X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fraenkel M, Gueorguiev M, Barak D, Salmon A, Grossman AB, Gross DJ. Everolimus therapy for progressive adrenocortical cancer. Endocrine. 2013;44(1):187–192. doi: 10.1007/s12020-013-9878-1. [DOI] [PubMed] [Google Scholar]
- 93.Szabó DR, Baghy K, Szabó PM, Zsippai A, Marczell I, Nagy Z, Varga V, Eder K, Toth S, Buzas EI, Falus A, Kovalszky I, Patocs A, Racz K, Igaz P. Antitumoral effects of 9-cis retinoic acid in adrenocortical cancer. Cell Mol Life Sci. 2014;71(5):917–932. doi: 10.1007/s00018-013-1408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]