Fig. 3.
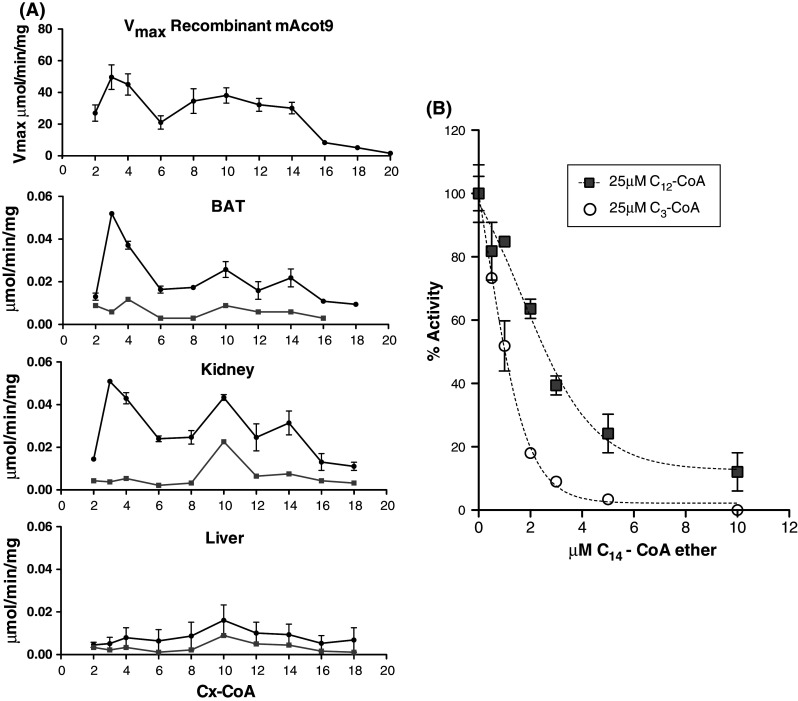
Acyl-CoA chain-length specificity of recombinant ACOT9 and thioesterase activity in isolated mitochondria. a Thioesterase activity of ACOT9 was measured spectrophotometrically at 412 nm with various concentrations of saturated acyl-CoAs and the V max values were calculated and plotted in the upper panel (mean ± SEM, n = 2–5). Mitochondria were isolated from BAT, kidney, and liver, sonicated and centrifuged, and thioesterase activity was measured in the supernatants as described under the "Materials and methods" section. The black circles show the activity with 50 μM of acyl-CoA esters ranging from C2 to C12-CoA, 25 μM C14-CoA and 10 μM C16-C18-CoA (data are mean ± SEM of two independent experiments). Note the very similar activity pattern for recombinant ACOT9 and BAT mitochondria. The grey squares show the remaining thioesterase activity after 2 min pre-incubation with 20 μM C14-CoA thioether with mitochondrial extracts. b C14-CoA thioether is a potent inhibitor of ACOT9 activity. Recombinant ACOT9 was pre-incubated with various concentrations of C14-CoA thioether (0–10 μM) and the activity was measured at fixed concentrations of C3-CoA (50 μM) (open circles) and C12-CoA (25 μM) (grey squares) using the spectrophotometric assay at 412 nm (mean ± SEM with two different enzyme preparations)
