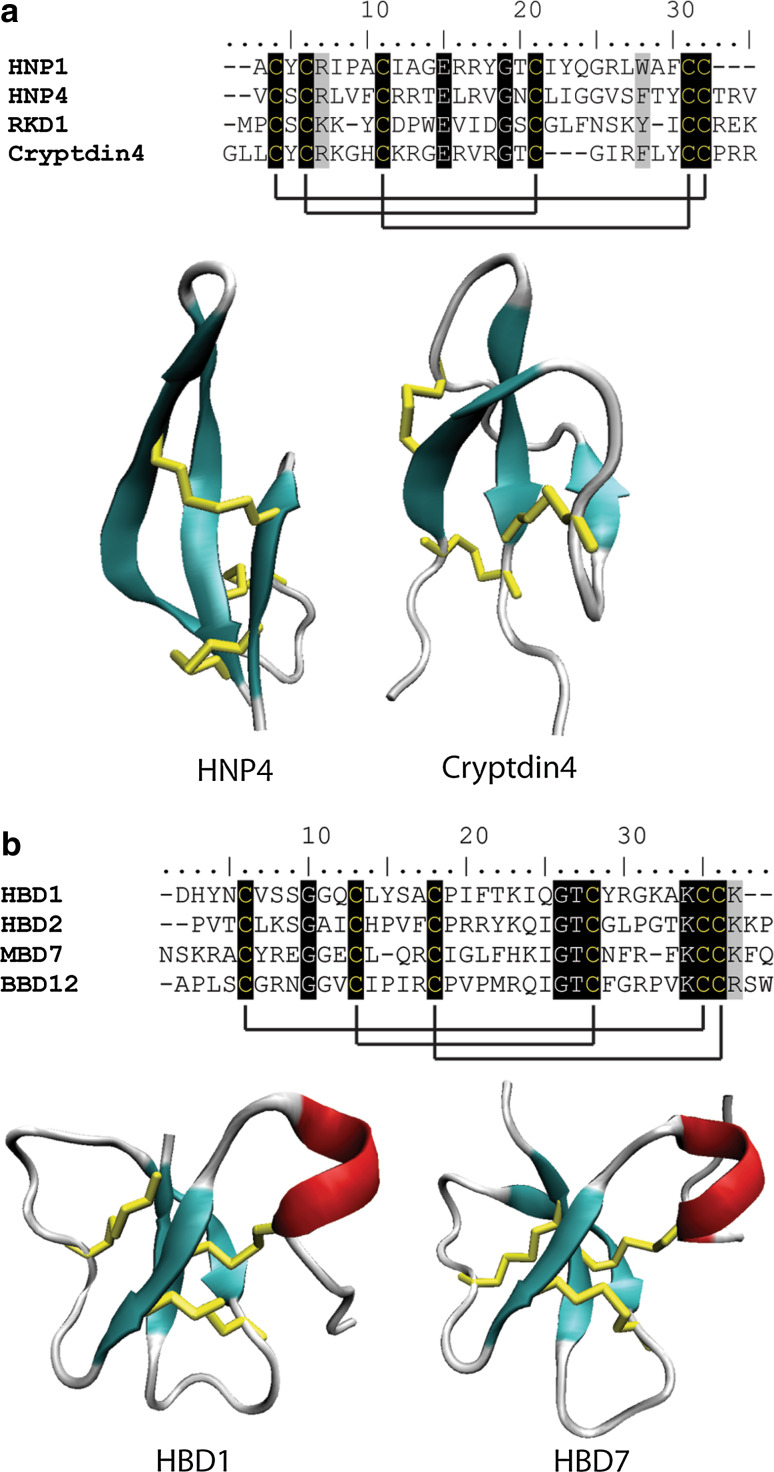
Fig. 7.
Sequence alignment and structural comparison of mammalian (a) α-defensins and (b) β-defensins. a Six cysteine residues which form three disulfide bonds are conserved in the three stranded β-sheet fold characteristic of mammalian α-defensins. Cysteine resides are highlighted in black with solid black lines denoting disulfide bonds. Residues highlighted in grey are highly conserved among the sequences HNP4 (pdb code 1ZMM) and cryptdin4 (pdb code 2GW9) form a triple-stranded anti-parallel β-sheet. b β-defensins contain six conserved cysteines that form three disulfide bonds although the connectivity of these disulfides differs to that of α-defensins. β-defensins also contain an additional short α-helix (HBD1, pdb code 1E4S and HBD2, pdb code 1E4Q). β-strands are represented in cyan, α-helices are in red, random coils and turns are in white, and disulfide bonds are in yellow