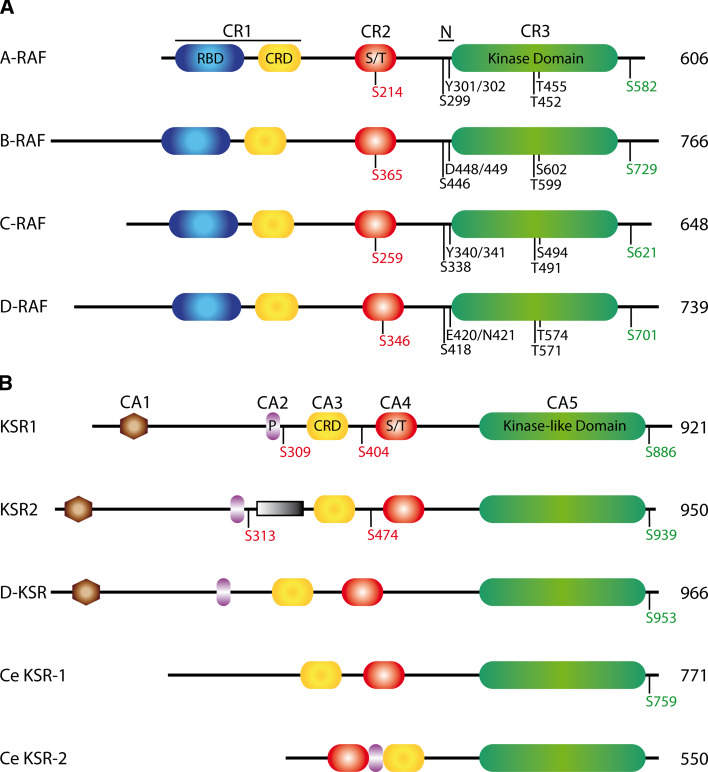
Fig. 1.
The domain organization of RAF (a) and KSR (b) proteins. Conserved regions (CR) and conserved areas (CA) are indicated at the top of each panel, as is the negative charge region (N) in the RAF proteins. Conserved phosphorylation sites are indicated underneath each isoform. Phosphorylation sites known or suspected of mediating binding to 14-3-3 proteins are colored red (negative regulation) or green (positive regulation). The size of each isoform is noted at the right. D-RAF and D-KSR are the Drosophila paralogs and Ce denotes C. elegans proteins. All other proteins depicted are from human. S309 and S404 in human KSR1 are the equivalent of S297 and S392 in mouse KSR1 (see text). The unique region of KSR2 is represented as a shaded box. CRD Cysteine-rich domain, P proline-rich, RBD RAS-binding domain, S/T serine/threonine-rich