Fig. 19.
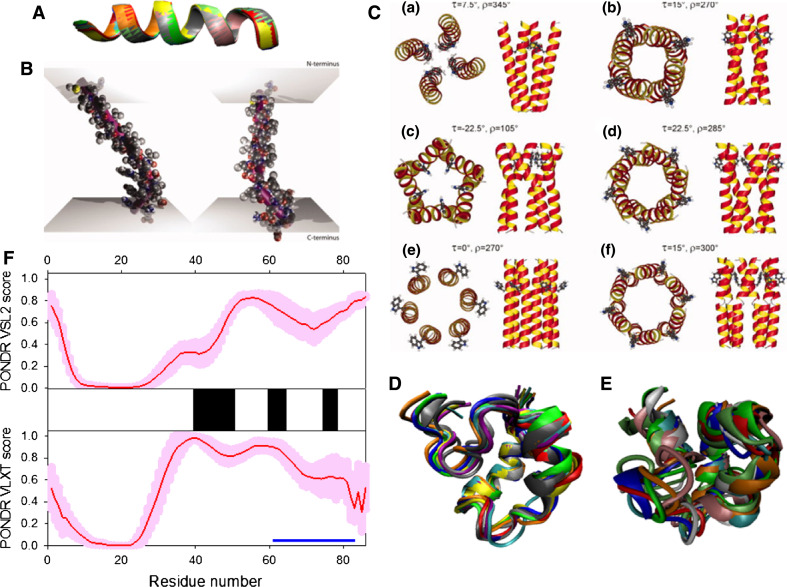
Disorder propensity and structural features of the HIV-1 viral protein U (Vpu protein). a NMR solution structure of the Vpu transmembrane domain (2GOH). Ten representative members of the conformational ensemble are shown by ribbons of different color. b Molecular dynamics (MD) simulation-based modeling of the average structure of the Vpu transmembrane domain within different membrane environments. The purple rods indicate the principal helical axis. On the left, the upper longer rod lies in the image plane, while the shorter rod is pointing away from the viewer. On the right the view point was rotated by 90°, allowing a view from the side. Reproduced with permission from Ref. [482]. c Models for the tetrameric (a, b), pentameric (c, d), and hexameric (e, f) Vpu TM oligomers that satisfy experimentally derived constraints. Reproduced with permission from Ref. [487]. d NMR solution structure of the cytoplasmic domain of Vpu in the presence dodecylphosphatidyl choline (DPC) micelles (2K7Y). Ten representative members of the conformational ensemble are shown by ribbons of different color. e NMR solution structure of the cytoplasmic domain of Vpu in aqueous solution (1VPU). Ten representative members of the conformational ensemble are shown by ribbons of different color