Fig. 6.
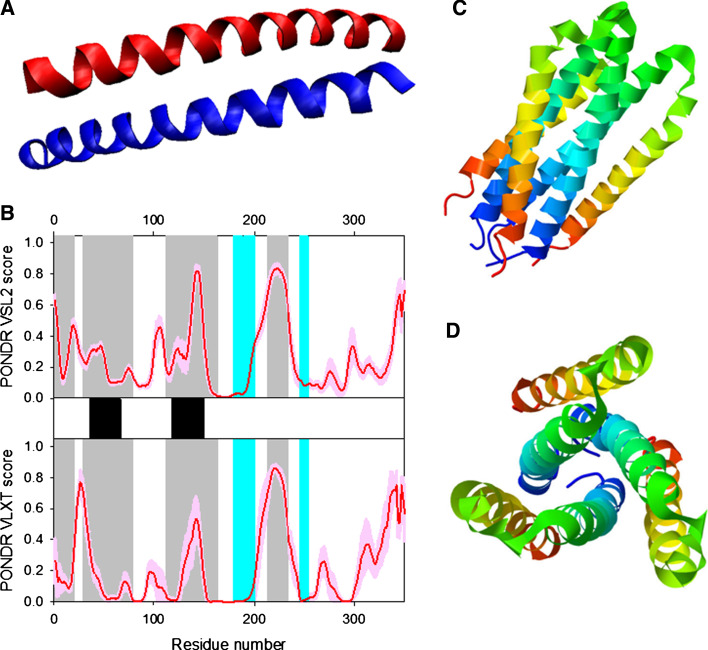
Disorder propensity and structural features of the HIV-1 gp41 protein. a The X-ray structure of the dimer between the N36 and C34 peptides forming the HIV-1 g41 core (1AIK). b Disorder prediction evaluated by PONDR® VSL2 (top panel) and PONDR® VLXT (bottom panel) for the HIV-1 gp41 protein. Red line represents an averaged disorder score for gp41 from ~50 different HIV-1 isolates. Pink shadow covers the distribution of disorder scores calculated for gp41 from these isolates. Locations of α-helices are indicated by black bars between the panels with the disorder scores. Gray shaded areas correspond to the functional domains of gp41, which, from left to right, are: the N-terminal hydrophobic glycine-rich “fusion” peptide; N51- and C43-peptides, and Kennedy sequence. c and d Side and top views of the crystal structure of a gp41 ectodomain core in its fusion-active state (1DF5). The structure is a six-helix bundle in which an N-terminal trimeric coiled coil is surrounded by three C-terminal outer helices in an antiparallel orientation