Fig. 2. Multimerization of ligand-free EphA2 is mediated by HH and HT interfaces.
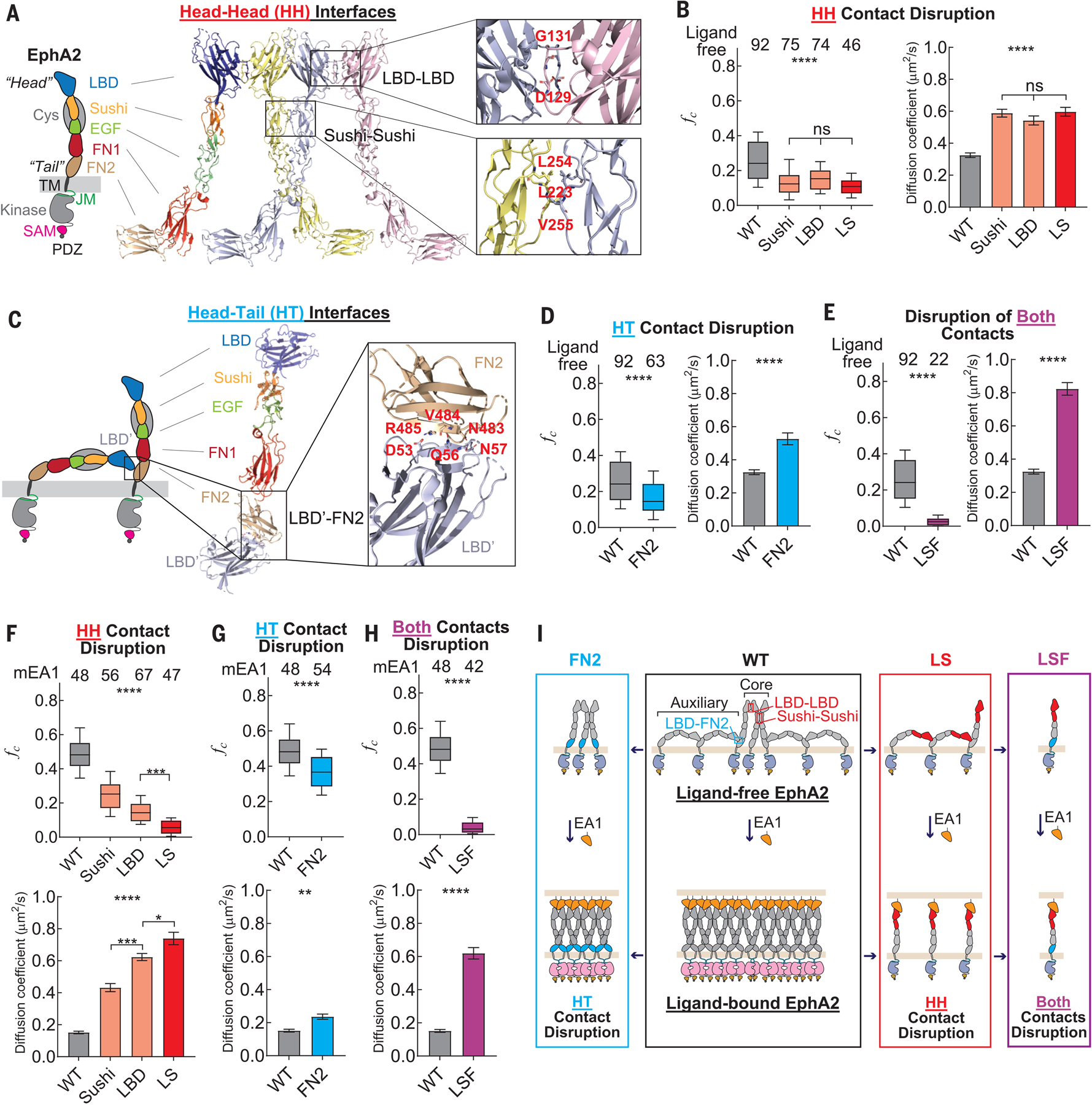
(A) Domain composition of the EphA2 receptor. The crystal structure of EphA2 ectodomain adapting HH contact through LDB-LBD and Sushi-Sushi interfaces is shown. Residues that mediate interactions are labeled. (B) fc values (left) and diffusion coefficients (right) of ligand-free EphA2 mutants that harbor a disruption at the HH interfaces. (C) Model of two ligand-free EphA2 molecules adapting HT contact through FN2 and LBD based on the crystal structure. Residues that mediate interactions are labeled. (D) fc values (left) and diffusion coefficients (right) of the ligand-free EphA2 mutant FN2, with disruption of the HT LBD-FN2 contact. (E) fc values (left) and diffusion coefficients (right) of the ligand-free EphA2 mutant LSF, with disruptions at both the HH and HT contacts. (F to H) fc values (top) and diffusion coefficients (bottom) of mEA1-stimulated EphA2 mutants with disruption at the HH interfaces (F), at the HT contact (G), and at both contacts (H). (I) Schematic diagram of the molecular assemblies of WT EphA2 (black box) and mutants that have disruption at HT (blue box), HH (red box), and both contacts (purple box). This diagram does not represent the exact numbers of EphA2 molecules in the molecular assemblies. In (B) and (D) to (H), the apparent diffusion coefficients are summarized in bar graphs and report the mean and SEM values. In the box plots, boxes represent third quartile, median, and first quartile, and the whiskers indicate the 10th to 90th percentile. Data were analyzed by one-way ANOVA and two-tail t tests; ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, and ns is not significant.
