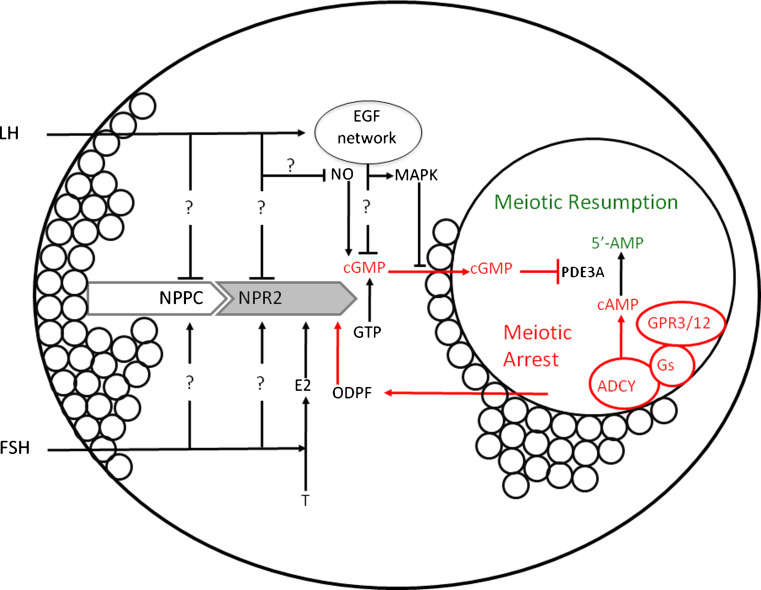
Fig. 1.
A proposed model for hormonal control of mammalian oocyte meiosis in pre-ovulatory follicle. NPPC produced by mural granulosa cells stimulates the generation of cGMP by cumulus cell NPR2, which diffuses into oocyte via gap junctions and inhibits oocyte PDE3A activity and cAMP hydrolysis and maintains meiotic arrest with a high intraoocyte cAMP level. This cAMP is generated through the activity of the Gs G-protein by GPR3/12 and ADCY endogenous to the oocyte. FSH, E2, and ODPFs may serve to maintain meiotic arrest by stimulating the expression of NPPC and/or NPR2 and the accumulation of cGMP, while the decrease of this cyclic nucleotide after LH treatment induces meiotic resumption by EGF network and/or by the down-regulation of NPPC and NPR2 expression. LH-induced peptides also activate MAPK and thus close the gap junctions throughout the somatic compartment, which reduces the flux of cGMP from the somatic cells to the oocyte. LH might also decrease nitric oxide level, and then decrease intraoocyte cGMP levels. FSH follicle-stimulating hormone, LH luteinizing hormone, EGF epidermal growth factor, MAPK mitogen-activated protein kinase, NO nitric oxide, T Testosterone, E2 17β-estradiol, ODPF oocyte-derived paracrine factor, NPPC natriuretic peptide precursor type C, NPR2 natriuretic peptide receptor 2, cAMP cyclic adenosine 3′,5′-monophosphate, cGMP cyclic guanosine 3′,5′-monophosphate, GPR3/12 G protein-coupled receptors 3 and 12, ADCY adenylyl cyclases, PDE3A phosphodiesterase 3A