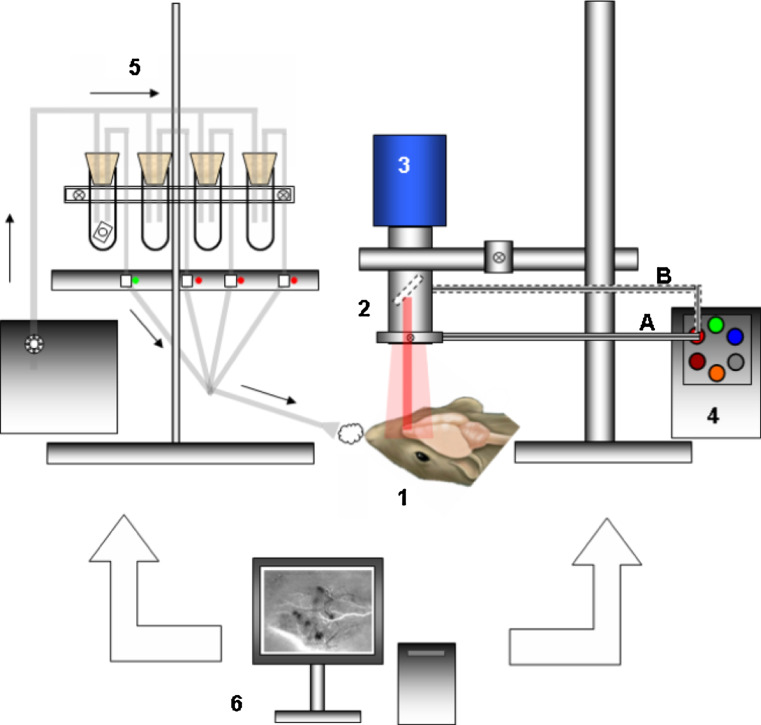
Fig. 4.
Wide-field optical imaging set-up for mapping odor-evoked activities in rodents. 1 Anesthetized animal with either full craniotomy or thinned bone above the MOB. Optical window is made of dental cement walls, agarose well and a microscope cover slip. 2 Wide-field microscopy optics with C-mount stereomicroscope or dual lens macroscope. 3 Cooled CCD camera, typically of 12-bit dynamics, with a frame rate of ten images per second or more at full frame. 4 Stabilized white light source with associated filter wheel. Light is shone onto the exposed brain tissue using a goose-neck optical light guide, using an annular fiber ring attached to the optics lens A, or through the epi-illumination port of the microscope B. Green light is used to visualize the blood vessel architecture. Bandpass interference filters are used to select the light wavelength for reflectance imaging or excitation of VSD or calcium dyes. For fluorescence studies the emission wavelength is selected using bandpass or long-pass filters placed in the detection path 5. Odors at a given concentration are deposited onto a filter paper and put into sealed vials (here only one odored paper is shown in the left vial in the figure). An olfactometer allows olfactory stimulation control and poststimulation air clean-up. In particular, the use of an air compressor with a manometer and computer-controlled electrovanes allows the mixing of several pure odorants if needed and stimulation for a given intensity and duration. 6 A computer allows the synchronization of illumination, olfactory stimulation and image acquisition. Activation maps are generally processed after acquisition but can also be displayed in video rate mode