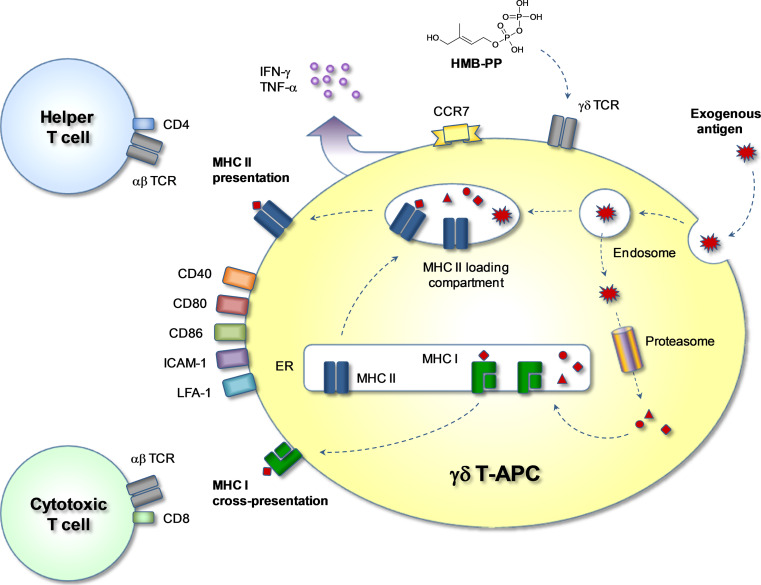
Fig. 2.
Antigen processing and presentation in γδ T-APCs. Exogenous soluble or particulate antigens are taken up by endocytosis and are directed to MHC I or MHC II loading pathways. By default, endosomal antigen reaches the lysosomal compartment where proteolytic degradation takes place. By vesicular fusion, some of the degraded antigen reaches the MHC II loading compartment where peptides are loaded onto newly formed MHC II molecules. Peptide-MHC II complexes are then relocated to the cell surface for presentation to CD4+ αβ T cells. Alternatively, by a process called antigen cross-presentation, antigen reaches the cytosol where the proteasome produces antigen-derived peptides, which are then transported into the endoplasmic reticulum (ER) to the site of newly formed MHC I molecules. Peptide-MHC I molecules are finally displayed on the cell surface for presentation to CD8+ αβ T cells. Activation of γδ T cells by HMB-PP leads to upregulation of co-stimulatory molecules such as CD40, CD80 and CD86, adhesion molecules such as LFA-1 (CD11a/CD18) and ICAM-1 (CD54), and the lymphoid homing chemokine receptor CCR7. Activated cells release cytokines such as IFN-γ and TNF-α, thus creating a pro-inflammatory microenvironment favoring the generation of CD4+ Th1 cells and cytotoxic CD8+ T cells