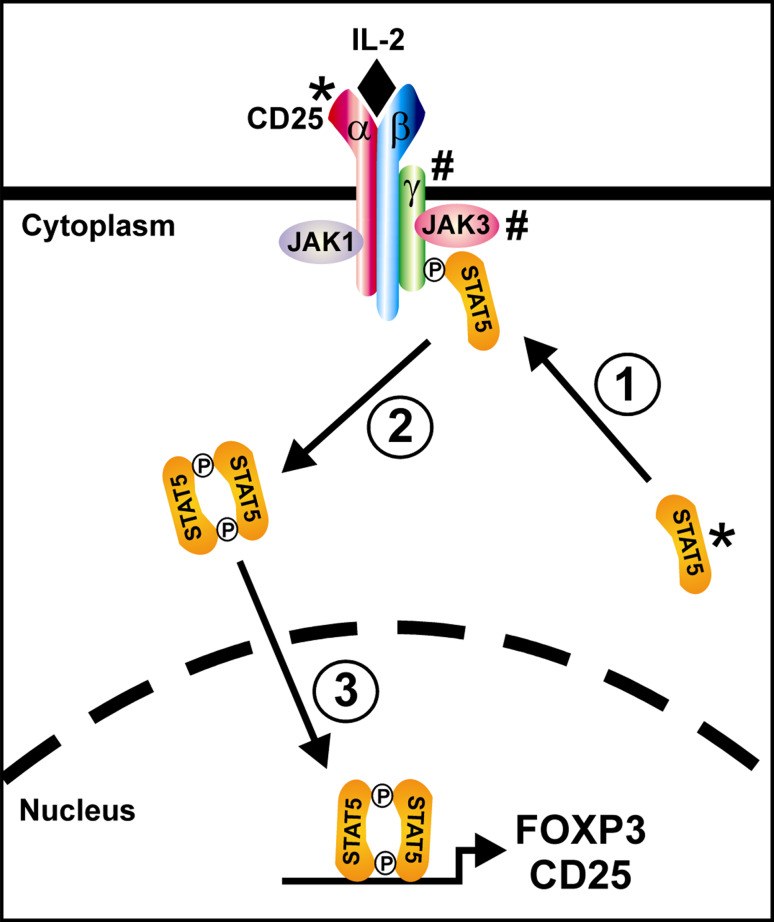
Fig. 1.
The role of CD25 and STAT5 in IL-2 signaling. The high-affinity IL-2 receptor is composed of α (CD25), β (CD122), and γ (γc; CD132) protein subunits together with receptor-associated tyrosine kinases of the JAK protein kinase family (JAK1 and JAK3). Upon binding of IL-2, the receptor subunits are clustered and phosphorylated on cytoplasmic tyrosine residues by the JAK kinases. Via its SH2 domain, unphosphorylated STAT5 binds to the phosphotyrosine residues on the activated receptor thus bringing it into proximity with the JAK kinases (1). The STAT5 subunits are then phosphorylated on a specific tyrosine residue by the JAK kinases causing them to dimerize (2), translocate to the nucleus, and bind DNA thereby upregulating expression of CD25, stabilizing expression of FOXP3, and modulating expression of a number of other gene targets (3). Note that defects in CD25 and STAT5 (marked by *) cause an immune dysregulation phenotype whereas defects in γc and JAK3 (marked by #) cause a phenotype of severe combined immune deficiency (SCID)