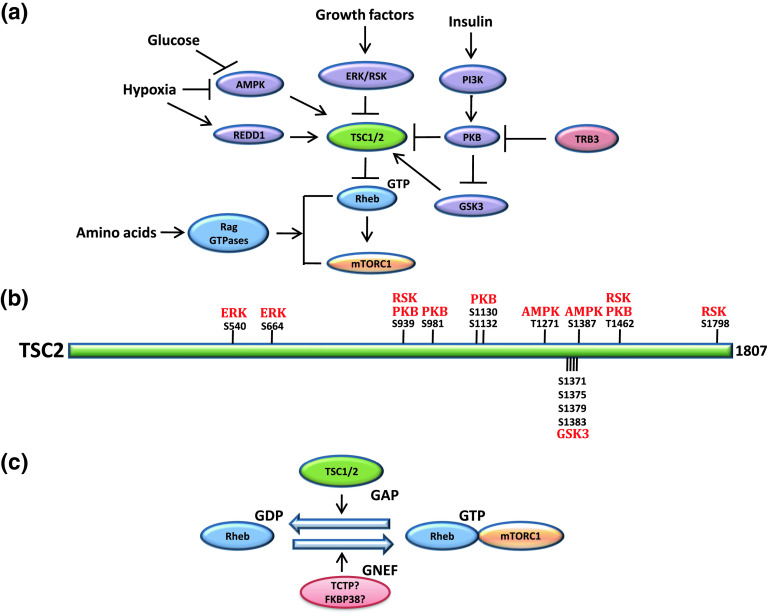
Fig. 2.
Schematic representation of signalling pathways that regulates mTORC1. a mTOR senses a wide range of upstream signals such as: amino acids availability, which modulates the activity of mTORC1 through Rag GTPases [88, 134]; glucose or oxygen levels through AMPK [AMP (5′-adenosine monophosphate) activated protein kinase] [78] and REDD1 (regulated in development and DNA damage responses 1) [18, 81, 149, 173]; growth factors, which activate MAPK (mitogen-activated protein kinase) and stimulate mTORC1 via ERK (extracellular signal-regulated kinases) and RSK (p90 ribosomal protein S6 kinase) [21, 22, 43, 101, 127, 128]; insulin via activation of PI3K (phosphoinositide 3-kinase), PKB (protein kinase B, also referred to as Akt) [52, 141], and inactivation of GSK3 (glycogen synthase kinase 3) [77] [n.b. the activity of PKB can be suppressed by TRB3 (mammalian homolog of Drosophila tribbles 3) [36]]. These pathways impinge on TSC1/2 (tuberous sclerosis complex 1/2), a GTPase activating protein (GAP) of the small G protein Rheb (Ras homolog enriched in brain), and GTP bound Rheb in turn activates mTORC1. b Sites of phosphorylation on TSC2 and their respective kinases, adapted from [73]. c The regulation of Rheb. TSC1/2 acts as a GAP for Rheb [139, 185], whereas TCTP (translationally controlled tumor protein) [71] and FKBP38 (FK506 binding protein 38) [4], have been proposed to act as a GNEF for Rheb