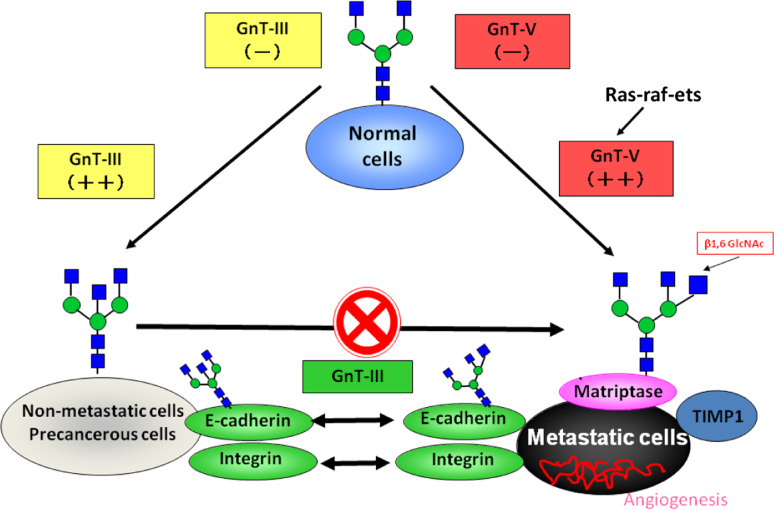
Fig. 5.
The role of N-glycan structures in the carcinogenic process. In normal cells, the GnT-III and GnT-V enzymes are normally underexpressed. The overexpression of GnT-III is associated with increased synthesis of bisecting GlcNAc structures in some important target glycoproteins involved in cell adhesion such as E-cadherin and integrins, the modification of which by bisecting N-glycans is associated with the suppression of metastasis through enhancement of E-cadherin-mediated cell–cell adhesion and a decrease in integrin-mediated cell-extracellular matrix adhesion. Furthermore, GnT-III up-regulation precludes the availability of the substrate for the GnT-V enzyme, which is no longer able to synthesize branched structures. In a metastatic cancer situation, activation of the ras-raf-ets signaling pathway regulates the transcription of the GnT-V gene and the resulting increase in GnT-V leads to increased enzymatic production of β1,6 branched structures that modify glycoproteins involved in the carcinogenic process, including Matriptase; TIMP-1 (Tissue Inhibitor of Metalloproteinase-1, in which β1,6 branching correlates with the invasive and metastatic potential of cancer cells) as well as integrins and E-cadherin, the modification of which contributes to a decrease in cell–cell adhesion, and increase in tumor cell invasion and migration. In addition, other mechanisms also indicate that a secreted type of GnT-V may contribute to tumor angiogenesis