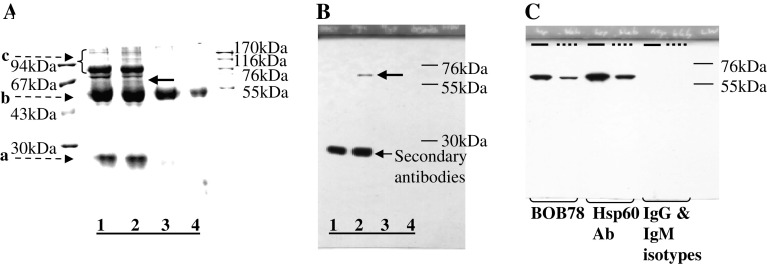
Fig. 1.
BOB78 antibody identifies rh-Hsp60. a, b BOB78 antigen was isolated from platelets by immunoprecipitation, and was revealed to be Hsp60 by sequencing. BOB78 antigen from the lysate of MEG-01-derived platelets was immunoprecipitated with BOB78 IgM monoclonal antibodies, complexed with rabbit anti-mouse antibodies, then captured by protein A Sepharose beads. Captured proteins were separated by SDS-PAGE and stained with Gel-Code. a Stained gel shows that BOB78 antigen was precipitated from the MEG-01 platelets lysate. There is a band specific to the reaction between BOB78 monoclonal antibody and the platelet lysate at 67 kDa (bold arrow, lane 2). This band was sent for sequencing by mass spectrometry (MALDI). Lanes 1, 3 and 4 represent control experiments: lane 1 BOB78 antibody supernatant, beads; lane 2 BOB78 antibody, platelet lysate, beads; lane 3 IgM isotype control, platelet lysate, beads; lane 4 platelet lysate, beads. Bands (broken arrows): a immunoglobulin light chain; b immunoglobulin heavy chain; c other proteins in supernatant. b Western blot probed with BOB78 antibody confirms the specificity of the BOB78 antigen band that was sequenced, as a single band at 67 kDa. The Western blot was performed on duplicate lanes corresponding to the immunoprecipitate gel in a . c The reactivity of BOB78 antibody for rh-Hsp60 protein was compared with that of commercial anti-Hsp60 monoclonal IgG. The lanes were loaded with either rh-Hsp60 (thick lines) or lysate from MEG-01 platelets (interrupted lines). The proteins were separated by SDS-PAGE and Western blotting was performed with BOB78, anti-Hsp60 monoclonal (Hsp60 Ab) and isotype control antibodies. Both the BOB78 antibody and the commercial anti-Hsp60 monoclonal antibody identify rh-Hsp60 and Hsp60 from MEG-01 platelets at 67 kDa. No band of identification is seen with the IgM and IgG isotype controls