Abstract
Neuronal migration is one of the most critical processes during early brain development. The gaseous messenger nitric oxide (NO) has been shown to modulate neuronal and glial migration in various experimental models. Here, we analyze a potential role for NO signaling in the migration of fetal human neural progenitor cells. Cells migrate out of cultured neurospheres and differentiate into both neuronal and glial cells. The neurosphere cultures express neuronal nitric oxide synthase and soluble guanylyl cyclase that produces cGMP upon activation with NO. By employing small bioactive enzyme activators and inhibitors in both gain and loss of function experiments, we show NO/cGMP signaling as a positive regulator of migration in neurosphere cultures of early developing human brain cells. Since NO signaling regulates cell movements from developing insects to mammalian nervous systems, this transduction pathway may have evolutionary conserved functions.
Keywords: Brain development, Neurospheres, Stem cells, Cell motility, Protein kinase G
Introduction
Neuronal migration during early phases of development and in some areas of the adult brain is important for the formation of functional neuronal networks. The gaseous messenger nitric oxide (NO), which is produced by the enzyme nitric oxide synthase (NOS), appears to play a critical role in proliferation, migration, and synaptogenesis during nerve cell development [1–3]. A major receptor for NO is the cGMP synthesizing enzyme soluble guanylyl cyclase (sGC), which has been implicated to be differentially expressed during neuronal development [4–6]. NO signal transduction has been shown to regulate the migration of cerebellar neurons, insect enteric neurons, and early developing Xenopus neuronal cells [7–10]. Neuroanatomical studies using markers against NOS and sGC identify the migrating neuroblasts of the rostral migratory stream as potential targets for NO signaling in the adult brain [11, 12].
The transient expression of NOS during development has provided additional evidence for the involvement of NO signaling in neuronal migration. For example, in the developing rat brain, neuronal NOS (nNOS) expression reaches the highest level between embryonic day 16 and postnatal day 0. This period corresponds to the migration of neuronal precursors from the ventricular zone to the external layers of the cortex [13, 14]. In the fetal human brain and spinal cord, some neurons express NOS as they migrate to their final destination [15, 16]. A recent study showed the expression of nNOS in cortical interneurons of fetal and infant human brain, which was either absent or substantially reduced in the congenital disorder holoprosencephaly [17]. Since cortical interneurons that populate the hippocampus and neocortex have to migrate from their site of developmental origin, expression of nNOS in such situation may suggest a critical role of NO in the formation of human brain architecture. Partly, due to ethical restrictions and limited availability of human fetal tissue, there is no functional evidence indicating the involvement of NO signaling in early developing human nerve cells.
In this study, we used a neurosphere assay of human neural progenitor cells (hNPCs) to investigate the role of NO/cGMP signal transduction in neuronal migration. Migrating hNPCs differentiate into neuronal and glial cells. Immunocytochemical methods demonstrate that hNPCs express nNOS and functional sGC. By employing small bioactive enzyme activators and inhibitors, we provide the first experimental evidence that NO/cGMP signal transduction positively regulates cell migration in developing human brain cells. The facilitatory role of NO signaling on cell movements in insects, amphibians, rodents [3, 7–10], and the hNPCs suggests evolutionary conserved developmental functions of this transduction pathway.
Materials and methods
NOC-18 [2,2′-(hydroxynitrosohydrazino) bis-ethanamine] was purchased from Calbiochem (Darmstadt, Germany), 8-Br-cGMP (8-Bromoguanosine 3′,5′-cyclophosphate) and Rp-8-Br-cGMP (8-Bromoguanosine-3′,5′-cyclophosphothioate, Rp-isomer) were purchased from Alexis Biochemicals (Lörrach, Germany). All other substances were obtained from Sigma (Taufkirchen, Germany) unless otherwise noted.
Cell culture
The hNPCs used in this study were purchased from Lonza Verviers SPRL (Verviers, Belgium) and were obtained from three individuals. The hNPCs were cultivated as previously described [18–20]. Briefly, cells were cultured in proliferation medium (Dulbecco’s modified Eagle's medium and Hams F12 (3:1) supplemented with B27 (Invitrogen GmBH, Karlsruhe, Germany), 20 ng/ml epidermal growth factor (Biosource, Karlsruhe, Germany), 20 ng/ml recombinant human fibroblast growth factor (R&D Systems, Wiesbaden-Nordenstadt, Germany), 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in a humidified 92.5% air/7.5% CO2 incubator at 37°C in suspension culture. Medium was changed every 2–3 days and spheres were chopped with a McIlwaine tissue chopper. Differentiation was induced by growth factor withdrawal in the presence of DFN medium [Dulbecco's modified Eagle's medium and Hams F12 (3:1) supplemented with N2 (Invitrogen)]. The neurospheres were cultured on chamber slides (Becton-Dickinson, Bedford, MA, USA) coated with poly-d-lysine and laminin, each at a concentration of 100 μg/ml. The NT2 cell line (American Type Culture Collection, VA, USA) was maintained as aggregate cultures in the presence of 10 μM retinoic acid for up to 2 weeks as previously described [21].
Migration assay
For the analysis of migration, chamber slides were initially coated with poly-d-lysine followed by laminin coating. The hNPCs were seeded on coated chamber slides at density of five neurospheres. The culture was treated with chemicals diluted in the differentiation medium for at least 72 h with a medium change after 48 h. The NO donor NOC-18, which decays with long half-life (about 57 h at 22°C) was prepared as a 100-mM stock solution in 10 mM of NaOH solution. As a solution, 200 mM 7NI (7-nitroindazole) and 20 mM ODQ (1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one) was prepared in DMSO (dimethylsulphoxide). 8-Br-cGMP Rp-8-Br-cGMP and Y27632 were directly dissolved into the medium. To determine the migration of neural progenitor cells out of neurospheres, images were acquired after 24 h of chemical application and migration distance was measured at four distinct locations from the rim of the spheres to the farthest migrated cells [19, 21]. After 72 h of migration, cultures were fixed and stained for cytoskeletal markers and migration was quantified at four distinct locations from the rim of the spheres to the farthest migrated doublecortin (DCX)-positive cells.
Cell viability/cytotoxicity assay
Cell viability was analyzed using the CellTiter-Blue assay (Promega, Mannheim, Germany) as previously described [19]. The assay is based on measurements of the mitochondrial reductase activity by conversion of the substrate resazurin to the fluorescent product resorufin, which can be assessed in a fluorometer. The lactate dehydrogenase (LDH) assay (CytoTox-One, Promega, Mannheim, Germany) was employed to assess cell death by measuring the amount of LDH, which is released into the medium from dead cells. The assay was performed according to the manufacturer’s instructions. Briefly, following 48 h of chemical exposure, supernatant of the culture medium (100 μl) was mixed with 50 μl of Cyto-Tox-One reagent and kept for 4 h in a 96-well plate. Fluorescence intensity was measured at excitation/emission wavelength of 540/590 nm. Results were presented as mean ± SEM of the percent maximum of LDH released from four measurements.
Immunocytochemistry
Immunocytochemical staining was performed following the procedures previously described by Tegenge and Bicker [21]. Briefly, cultures were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Primary antibodies against βIII-tubulin (Sigma, 1:10,000), nestin (Calbiochem, 1:400), glial fibrillary acidic protein (GFAP, Sigma, 1:500) and doublecortin (DCX, Santa Cruz Biotechnology, Inc., CA, USA, 1:500) were applied for 2 h at room temperature. Secondary biotinlyated antibodies (Vector, Burlingame, MA, USA) were applied for 1 h. Immunofluorescence was detected by applying streptavidin-CY3 (Sigma) or streptavidin-Alexa Fluor 488 (Mobitec, Göttingen, Germany) for 1 h. DAPI (4′,6-diamidino-2′-phenylindoldihydrochloride) at 1 μg/ml was used as a nuclear counterstain.
Detection of NO-sensitive sGC
For the detection of cGMP immunoreactive (cGMP-IR) cells, hNPCs were preincubated for 20 min at 37°C with 1 mM sodium nitroprusside (SNP) as a NO donor, 20 μM YC-1 (3-(50-hydroxymethyl-20-furyl)-1-benzyl indazole) as an enhancer of NO-induced activity of sGC, 50 μM of ODQ as a sGC inhibitor and 1 mM IBMX (3-isobutyl-1-methylxanthine) as a phosphodiesterase inhibitor. Afterwards, cultures were washed once with PBS and fixed with 4% PFA. The polyclonal sheep cGMP antiserum (1:10,000; a kind gift from Dr. J. de Vente, Maastricht University, Netherlands) was used as primary antibody.
Western blotting
The protein content of the cell lysate was estimated using the BCA™ protein assay kit (Pierce, Rockford, IL, USA). About 50 μg of protein from proliferating hNPCs was subjected to electrophoresis on 8% SDS acrylamide gel and Western blotting was carried out as previously described [21]. The monoclonal anti-nNOS (Sigma) at 1:1,000 dilution was used to identify endogenous sources of NO synthesis. Monoclonal anti-acetylated-α-tubulin (Sigma) diluted 1:10,000 were used as a loading control.
Determination of nitrite
Dose-dependent accumulation of NO in culture from a NO donor (NOC-18) was estimated from the quantification of nitrite using a high-sensitivity nitrite assay kit (Molecular Probes, Eugene, OR, USA). The assay was performed according to the manufacturer’s instructions.
Microscopy and data analysis
Preparations were examined with a Zeiss Axiovert 25 fluorescence microscope equipped with an Axiocam 3900 digital camera or Zeiss Axio Observer D1 (Zeiss, Göttingen, Germany). The percentage of nestin, βIII-tubulin, GFAP, and cGMP-positive cell bodies was determined by dividing the number of positively stained cells by the total number of cells in the migration area. The data were presented as mean ± SEM of at least three independent experiments. Statistical comparisons of control versus treatment were performed with the unpaired two-tailed Student’s t test. Levels of significance are indicated as *p < 0.05, **p < 0.01 and ***p < 0.001.
Results
Nitric oxide-sensitive sGC is expressed in early developing human brain cells
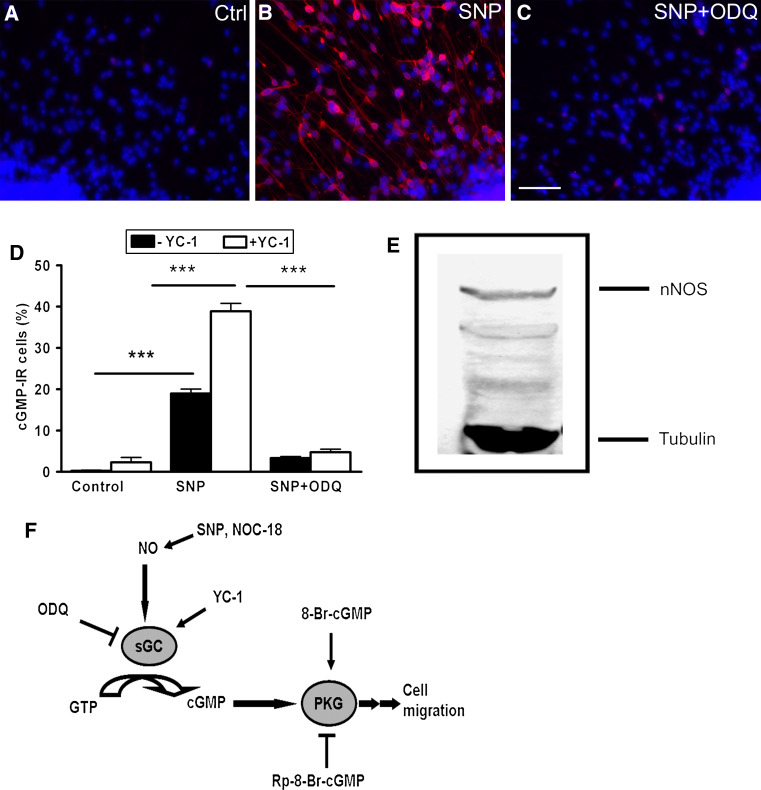
An antibody against cGMP was used to detect NO-sensitive sGC that synthesizes cGMP upon stimulation [22]. In the absence of exogenous NO, only a few cGMP-IR cells were detected even in the presence of YC-1, a haem-dependent stimulator of sGC (Fig. 1a, d). The number of cGMP-IR cells was increased significantly up to 19 ± 1% upon stimulation with the NO donor, SNP. In the presence of YC-1, sGC activation by NO was potentiated (Fig. 1b, d). Several mechanisms have been proposed for NO-induced potentiation of sGC activity by YC-1, which includes stabilization of the nitrosyl-haem complex, transformation of NO-activated enzyme from a low- to high-output activation state and inhibition of cGMP-metabolizing phosphodiesterases [23]. The NO-induced cGMP-IR cells were significantly reduced when SNP stimulation was accompanied by the sGC inhibitor, ODQ (Fig. 1c, d). On Western blots of proliferating hNPCs, anti-nNOS monoclonal antibody labeled a major protein band around 155 kDa (Fig. 1e), indicating that nitric oxide could be endogenously produced in the neurosphere culture. In developing human fetuses, NOS expression has been reported in subpopulations of migratory-like neurons within the subplate zone and the cortical plate [15, 17]. In order to identify the cellular source of NO in our neurosphere culture, we used antibody directed against the nNOS and a conserved sequence within the three isoforms of NOS. However, due to a weak and rather homogeneous immunoreactivity we were not able to identify any differential staining of NOS in the heterogeneous cell population of the human neurosphere (data not shown).
Fig. 1.
Functional NO/cGMP signaling in early developing human nerve cells. The presence of NO-sensitive sGC was demonstrated by immunocytochemical detection of cGMP. The hNPCs were allowed to migrate and differentiate on poly-d-lysine/laminin-coated substrates for 72 h. Cultures were exposed for 20 min to a 1 mM IBMX + 20 μM YC-1 (control), b SNP (1 mM), or c SNP (1 mM) + ODQ (50 μM) together with IBMX + YC-1. Cultures were fixed with 4% PFA and stained against cGMP. d The cGMP-IR cells were counted under control condition and after stimulation with NO donor (SNP) with or without YC-1. e The antibody against nNOS recognizes a protein band of apparent molecular weight at 155 kDa from the proliferating hNPCs on Western blot. The lower band represents acetylated-α-tubulin. f Schematic drawing of NO/cGMP signal transduction together with an array of activators and inhibitors of the pathway. Data are mean ± SEM of ten neurospheres from at least two independent experiments. Scale bar 50 μm
cGMP-IR progenitor and glial cells direct the migration out of the neurospheres
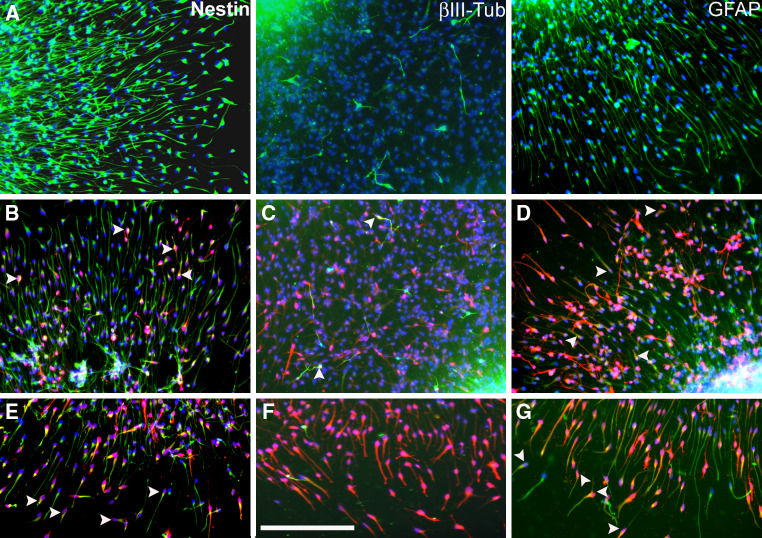
Upon withdrawal of growth factor, cells migrated radially out of the sphere on PDL/laminin-coated surface, thereby forming a migration area that increased with time. After 24 h of plating, almost all migrated cells appeared to express nestin, indicating that the neural progenitor cells were the first to migrate out of the spheres [data not shown, 24]. This neural progenitor migration was comparable in hNPCs obtained from three different individuals [24]. Here, the progress of neuronal differentiation within hNPCs was monitored by immunocytochemical staining against cytoskeletal markers of progenitor cells (nestin), early neurons (βIII-tubulin), and glial cells (GFAP) after 72 h of plating. The majority of the cells that migrate out of the neurospheres were positively stained for nestin, GFAP and with a few interspersed βIII-tubulin stained neurons (Fig. 2a). In order to address which cell types would respond to stimulation by NO, we co-stained cGMP with cytoskeletal markers. Our results indicated that about 53.5 ± 2% of the cGMP producing cells were GFAP-IR while the nestin and βIII-tubulin-IR cells constituted about 21 ± 3 and 9.6 ± 1%, respectively (Fig. 2b–d). The mainly nestin and GFAP expressing cells at the forefront of migration did double-label for cGMP (Fig. 2e–g).
Fig. 2.
Immunocytochemical characterization of hNPCs for cytoskeletal markers and NO signal transduction. a After 72 h of migration on poly-d-lysine/laminin-coated chamber slides, cells that migrated out of the neurospheres express mainly nestin and GFAP with few interspersed βIII-tubulin-IR cells. After exposure of hNPCs to SNP + IBMX + YC-1 for 20 min, the cultures were co-stained for cGMP and b nestin, c βIII-tubulin, or d GFAP. e cGMP-IR cells at the forefront of migration were co-labeled for nestin. f cGMP producing cells at the forefront of migration lack βIII-tubulin staining. g cGMP-IR cells at the forefront of migration were co-labeled for GFAP. Blue (a–g) indicate DAPI nuclear staining. Dense cellular aggregate staining (a–d) delineates the edge of the neurospheres. Arrow heads (b–g) show representative co-localization of cGMP with respective cytoskeletal markers. Scale bar 50 μm
Blocking NO and cGMP production retards neural progenitor cell migration
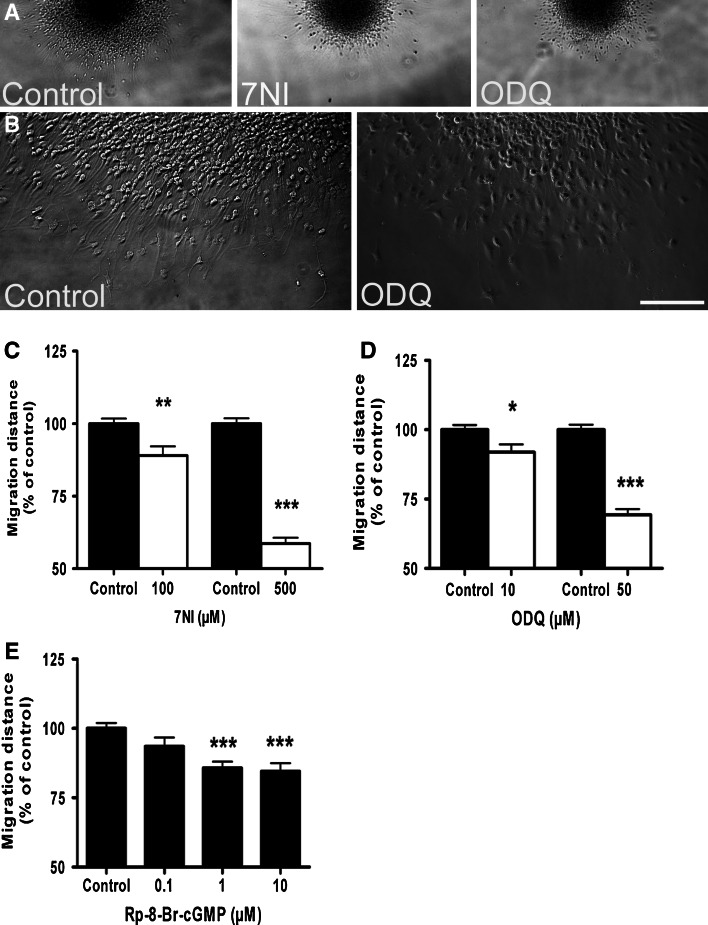
Next, we tested whether NO signaling modulates the migratory behavior of hNPCs. The concentration of both inhibitors and activators of the NO/cGMP/PKG pathway (Fig. 1f) were selected based on previous migration experiments using differentiating NT2 neurons [21]. Application of inhibitors of nNOS (7NI) and sGC (ODQ) significantly reduced the migration of progenitor cells out of neurosphere (Fig. 3a–d). In the presence of PKG-I inhibitor (Rp-8-Br-cGMP), cell migration was reduced in a dose-dependent manner (Fig. 3e).
Fig. 3.
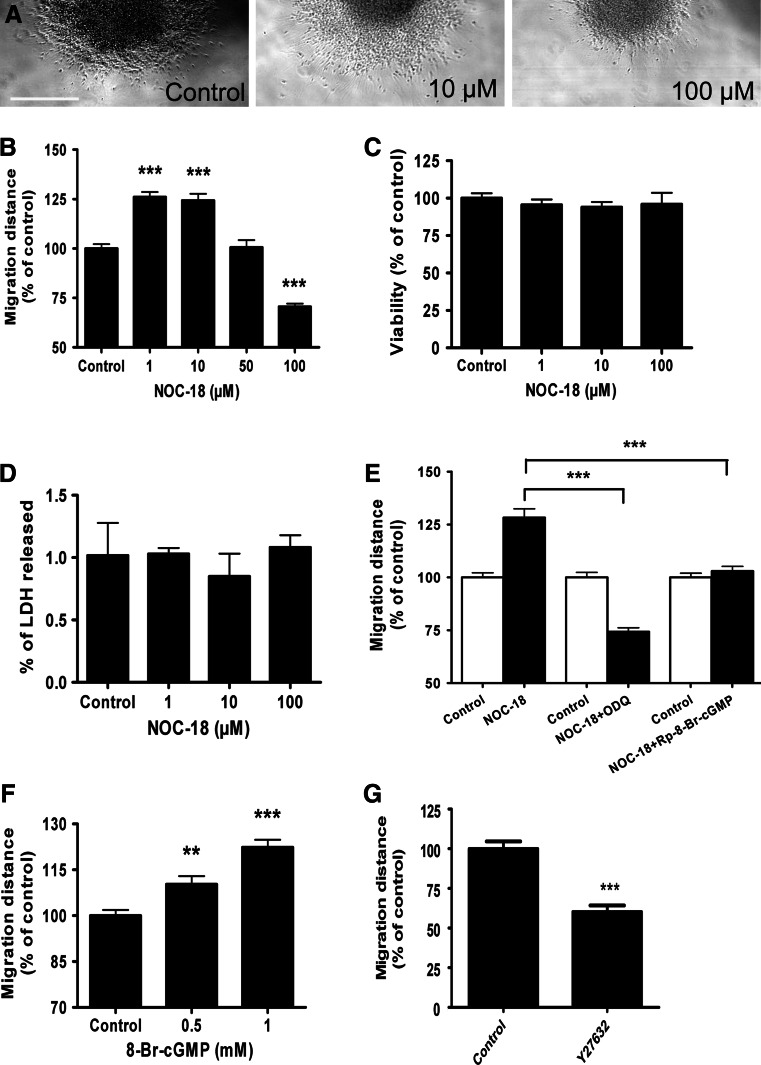
The nNOS/sGC/PKG enzyme inhibitors block the migration of hNPCs. a Representative photomicrograph of the neurospheres at upper edge of figure after 24 h of migration under control condition (0.25% DMSO) and in the presence of nNOS (7NI, 500 μM) and sGC (ODQ, 50 μM) inhibitors. b Higher magnification of cells in the migration area. c–e Migration distance from the rim of the sphere to the furthest migrated cells was quantified at four distinct locations per sphere. Application of c 7NI, d ODQ, and e Rp-8-Br-cGMP significantly blocked cell migration in a dose-dependent manner. Data represent mean ± SEM of at least ten neurospheres from three independent experiments. Scale bars 500 μm (a) and 250 μm (b)
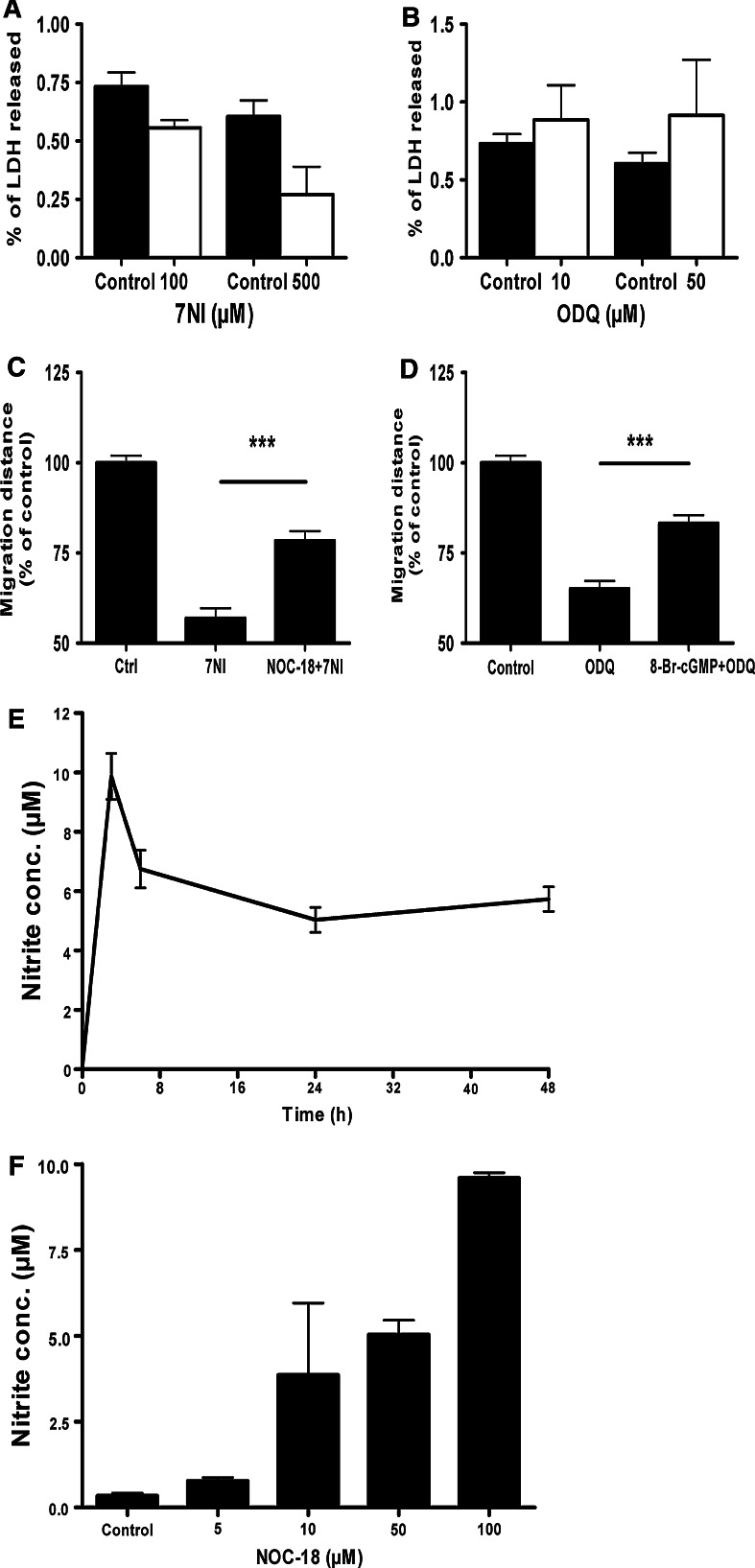
A LDH cytotoxicity assay was conducted after the application of enzyme inhibitors of nNOS and sGC. Both 7NI and ODQ did not significantly alter the viability of cells at the concentrations used to inhibit cell migration (Fig. 4a, b). Application of NOC-18, which releases NO in the culture, partially rescued the blocking of cell migration caused by nNOS inhibition (Fig. 4c). Similarly, a cell membrane permeable analogue of cGMP, 8-Br-cGMP, partially rescued cell migration that was blocked by ODQ (Fig. 4d).
Fig. 4.
Blocking of cell migration by nNOS and sGC inhibitors is not caused by cytotoxic effects. a 7NI and b ODQ did not cause cytotoxicity as determined by the level of LDH released. Exogenous application of c NO and d cGMP rescue the blocking of cell migration by nNOS and sGC inhibitors, respectively. e Release profile of NO from 50 μM of NOC-18 as determined by the level of nitrite. f Application of NOC-18 for 24 h result in accumulation of NO in a dose-dependent manner. Data represent mean ± SEM of four measurements (a, b, e and f) and at least ten neurospheres (c and d) from three independent experiments
NO and cGMP facilitates cell migration
We initially tested the kinetics of NO production from the donor (NOC-18) that released nitrite in a dose-dependent manner for over 48 h (Fig 4e, f). Exposure of hNPCs to NOC-18 (1–10 μM) for 24 h significantly facilitated the migration of neural progenitor cells out of the spheres (Fig. 5a, b). However, 100 μM of NOC-18 inhibited cell migration (Fig. 5a, b). At this high concentration, NOC-18 might affect cell viability. Hence, we assessed cell viability/cytotoxicity using the CellTiter-Blue and LDH assay in parallel to the migration experiments. Both methods showed no sign of cytotoxicity upon NOC-18 application, even at higher concentration that had blocked cell migration (Fig. 5c, d). To directly test whether NO facilitates cell migration via its potential downstream target enzymes, 10 μM of NOC-18 was co-applied with sGC or PKG inhibitors. A significant reduction of NO-induced cell migration was observed when NOC-18 was used in combination with ODQ or Rp-8-Br-cGMP (Fig. 5e). Application of the cGMP analogue 8-Br-cGMP significantly facilitated the migration of cells (Fig. 5f). Changes in cell motility induced by cGMP/PKG signaling are often mediated by small Rho GTPases including RhoA and its major downstream effector Rho Kinase (ROCK). Here, the use of ROCK inhibitor Y27632 significantly blocked cell migration (Fig. 5g).
Fig. 5.
NO donor and cell membrane permeable analogue of cGMP facilitates cell migration. a Representative photomicrographs of hNPCs after 24 h of migration under control condition and in the presence of 10 μM NOC-18 and 100 μM NOC-18. b The migration of cells increased significantly in the presence of 1–10 μM of NOC-18. The viability of cells was not affected by NOC-18 application as determined by (c) CellTiter-Blue assay and (d) percent LDH released. e Co-application of NOC-18 (10 μM) together with down-stream enzyme inhibitor of sGC (ODQ, 50 μM) or PKG (Rp-8-Br-cGMP, 1 μM) reduced NO-induced migration of cells. f 8-Br-cGMP facilitated cell migration. g ROCK inhibitor Y27632 (50 μM) blocks cell migration. Data represent mean ± SEM of at least 15 neurospheres (b, e, f, and g) and four measurements (c and d). Scale bar 500 μm
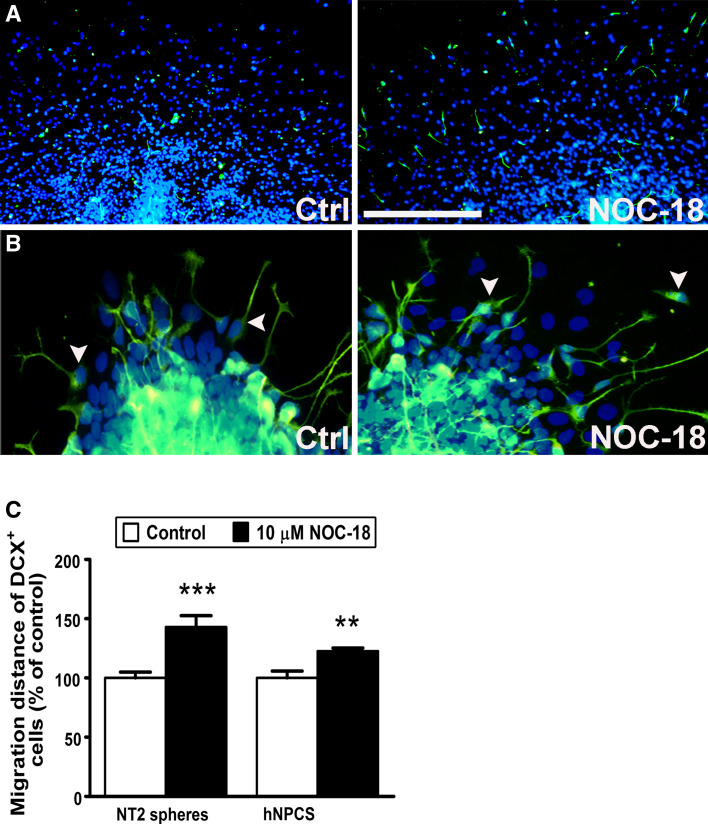
So far we have studied NO/cGMP signaling as a regulator of neuronal progenitor cell migration. We asked whether the migration of differentiated neurons was also influenced by NO. For this purpose, we used doublecortin (DCX) as an early marker of migrating immature neurons. Here, we used a widely used human neuronal precursor cell line (NT2 cells) in addition to the hNPCs. After 72 h of migration and differentiation some cells in the migration area of the hNPCs expressed DCX. In comparison, we found that most of the migrating cells from NT2 cell spheres expressed DCX after 2 weeks of neuronal differentiation. Application of NOC-18 for 72 h facilitated migration of DCX-positive cells both from cultured hNPCs and NT2 spheres (Fig. 6a–c). Taken together, our data indicate that NO enhances the migration of both neural progenitor cells and immature neurons.
Fig. 6.
NO facilitates the migration of doublecortin (DCX)-positive immature neurons. Application of 10 μM of NOC-18 for 72 h in a hNPCs and b human NT2 cell aggregates enhances migration of DCX-expressing cells. NT2 cells were treated with retinoic acid for 2 weeks in aggregate culture. c Migration distance of DCX-positive cells was measured from the rim of the spheres. Arrowheads indicate representative DCX-positive migrating cells. Data represent mean ± SEM of at least eight spheres from two independent experiments. Scale bar 50 μm (a) and 100 μm (b)
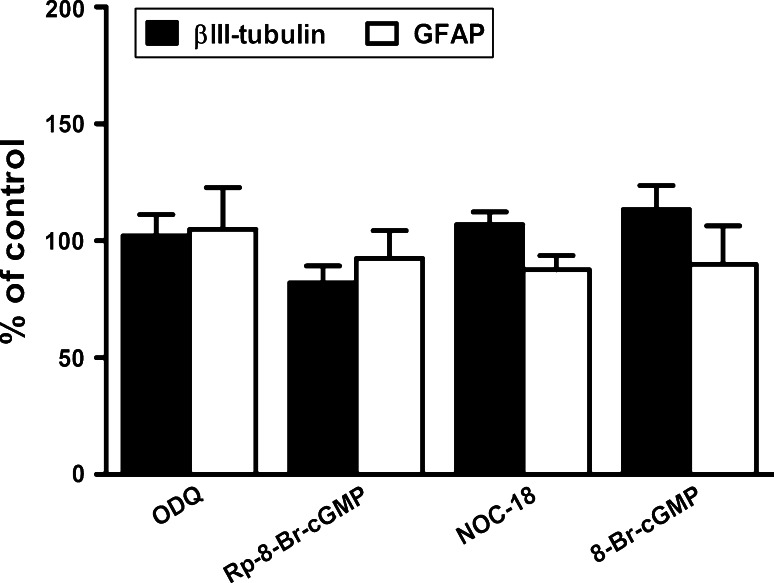
Finally, we counted the number of βIII-tubulin-IR and GFAP-IR cells to examine the potential effects of bioactive ligands of the sGC/PKG pathway on differentiation. At the concentration used to modulate cell migration, none of the chemicals significantly altered the proportion of neuronal and glial cells (Fig. 7).
Fig. 7.
The proportion of neuronal and glial cells was not changed in the presence of chemicals that modulate cell migration. Application of ODQ (50 μM), Rp-8-Br-cGMP (1 μM), NOC-18 (10 μM), or 8-Br-cGMP (1 mM) for 72 h with a medium change after 48 h did not significantly alter the percentage of βIII-tubulin and GFAP immunoreactive cells. Data are mean ± SEM of 15 neurospheres from three independent experiments
Discussion
Directed cell migration is an important biological process during embryogenesis, immune response, angiogenesis, and neuronal development. Cell migration is also important in the progression of diseases that include tumor cell invasion, inflammation, and congenital brain defects [25]. In this study, we identify NO/cGMP signaling as positive regulator of hNPCs migration.
Migrating nestin and GFAP-positive cells constitute the major portion of cultured human neurospheres within 72 h of differentiation. Nevertheless, upon prolonged time of culturing (7–14 days), most of the progenitor cells have been demonstrated to differentiate into βIII-tubulin-IR neurons [24, 26]. Since hNPCs generate both neuronal and glial cells, with the latter in a higher proportion and at the forefront of migration (Fig. 2g), the neurons appear to migrate along a glial scaffold. Indeed, real-time microscopic observation for 24 h revealed that cells migrate out of human neurospheres mainly through a radial course [24]. Thus, the use of hNPCs to study cell movements may resemble aspects of neuroblast migration along radial glia that constitutes major migratory pathway during early development of the brain [27]. This feature makes the hNPC neurosphere culture a more realistic model of migratory mechanisms in human brain development than the previously described NT2 cell spheres [21], which differentiated mainly into neurons.
Several experimental findings show that NO/cGMP signaling is crucial for human neural progenitor migration. Firstly, the migrating human cells synthesize cGMP upon stimulation with NO donor indicating that NO-sensitive sGC is expressed in developing human brain cells. Co-staining of cGMP with cytoskeletal markers in the whole migration area and at the forefront of migration showed that a major proportion of cGMP-producing cells were the GFAP and nestin-positive neuronal progenitor cells (Fig. 2e–g). Thus, the migrating precursor and glial cells are responsible for the synthesis of cGMP upon stimulation with NO. These migratory cells are trailed by βIII-tubulin-IR neuronal cells (Fig. 2c, f).
Secondly, application of enzyme inhibitors of the nNOS/sGC/PKG pathway in hNPCs culture significantly reduced the migration of cells out of the neurospheres (Fig. 3). This loss of function effect and the presence of cGMP-IR cells at the forefront of migration infer that a certain level of cGMP is required to facilitate the migration of progenitor cells out of the neurospheres. Morphological observation of neurospheres and the LDH assay revealed no sign of cytotoxicity due to the chemical inhibitors. Moreover, application of an NO donor and cGMP analogue partially rescued the blocking of cell migration caused by nNOS and sGC inhibitors, respectively. Thus, it is unlikely that unspecific side effects of the chemical blocker contribute to inhibition of cell migration.
In gain-of-function experiments, exposure of hNPCs to the NO donor, NOC-18 (1–10 μM), and cGMP analogue significantly facilitated the migration of cells out of the neurospheres. This experimental approach is in line with our recent finding on the retinoic acid induced differentiating human neuronal precursor (NT2) cells. In NT2 cells, NOC-18 facilitated cell motility in a concentration range of 1–100 μM, above which we observed deleterious effects on cell viability and proliferation [21]. Here, 100 μM of NOC-18 significantly inhibited cell migration without altering cell viability. In comparison to the migrating NT2 cells, the hNPCs seemed to be about ten times more sensitive to the NO donor. The inverted U-shaped dose-response curve (Fig. 5b) suggests that NO is playing a dual role, facilitating cell migration at low concentrations while slowing it down at higher concentrations. Similar concentration-dependent effects on growth cone motility have been observed on developing snail neurites [28] and migrating neutrophils [29]. Further evidence for the requirement of sGC and PKG in the facilitating migration at low concentrations of NO, was provided by co-application of NOC-18 with ODQ or Rp-8-Br-cGMP.
The detailed mechanism by which cGMP-activated PKG facilitates cell motility is presently unknown. In both neuronal and glial cells, reorganization of actin cytoskeleton is caused downstream of the cGMP pathway [8, 30], which may involve inhibition of RhoA GTPase [30–32]. The PKG substrate enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) family proteins that regulate actin polymerization have also been suggested to mediate the action of cGMP during cell motility [33–35]. In our human neural progenitor cultures, cell migration was influenced by inhibitors of sGC/PKG (Fig. 3d, e) and ROCK enzymes (Fig. 5g). The detailed molecular components linking NO/cGMP signaling to cytoskeletal rearrangements require further investigation.
Even though targeted deletion of nNOS isoform in mice seems to cause no major neuroanatomical defects [36], our cell culture experiments indicate a critical role for NO and cGMP in regulating human neural progenitor migration. This discrepancy may be explained by compensatory mechanisms in the developing rodent brain. Moreover, species-specific differences in the cellular expression of NOS during development [15] and a different arrangement of migratory pathways of interneuronal precursors in the human brain [17, 27] could account for our findings.
It is perhaps not too surprising that NO may function as regulator of cell motility in the developing nervous system, since it has already been recognized as an essential mediator of migration in smooth muscle cells and macrophages [29, 37, 38]. Based on modeling studies, NO has been hypothesized to serve as a rapidly diffusible signal in activity-dependent morphogenetic processes during the formation and functioning of the brain [39]. There is now converging experimental evidence from several animal models that NO participates in the two early developmental processes of cell proliferation and migration [1–3, 7–10, 40]. NO as a diffusible volume messenger may be particularly well suited for the complex spatio-temporal coordination of cell proliferation and morphogenetic movements during early development [9, 41]. In this study, we discovered that NO/cGMP signaling positively regulates the migration of primary fetal human progenitor cells culture during neuronal differentiation. Since transcellular NO/cGMP/PKG signaling also enhances neuronal cell migration in a wide range of developing nervous system [3, 7–10] this transduction cascade may have evolutionary conserved functions in nervous system development.
In the adult mammalian brain, the migrating neuroblasts of the rostral migratory stream have been suggested as a putative target for NO/cGMP signaling [11, 12]. In a model of ischemic stroke, NO donor has been shown to facilitate neuroblasts migration in the subventricular zone and dentate gyrus [42, 43]. Thus, the stimulatory effects of NO signaling may be relevant to cell motility during early human brain formation, as well as to the development of therapeutic strategies to mobilize neuroblast migration in adult tissue after brain injury.
Acknowledgments
We thank Dr J. de Vente for his kind gift of the cGMP antiserum, Dr. M. Stern for the discussion during the preparation of the manuscript, and Nadine Seiferth for technical support. We also like to acknowledge the contribution of Susanne Giersiefer to the experiment with the ROCK inhibitor. We thank Prech Uapinyoying for reading and correcting the manuscript. M.A. Tegenge received a Georg-Christoph-Lichtenberg scholarship from the Ministry for Science and Culture of Lower Saxony. This work was supported by the BMBF grants (013925C) to E. Fritsche (013925D) to G. Bicker and DFG grant (FG 1103, BI 262/16-1).
Contributor Information
Ellen Fritsche, Phone: +49-211-3389217, FAX: +49-211-3190910, Email: ellen.fritsche@uni-duesseldorf.de.
Gerd Bicker, Phone: +49-511-8567765, FAX: +49-511-8567687, Email: gerd.bicker@tiho-hannover.de.
References
- 1.Enikolopov G, Banerji J, Kuzin B. Nitric oxide and Drosophila development. Cell Death Differ. 1999;6:956–963. doi: 10.1038/sj.cdd.4400577. [DOI] [PubMed] [Google Scholar]
- 2.Cárdenas A, Moro MA, Hurtado O, Leza JC, Lizasoain I. Dual role of nitric oxide in adult neurogenesis. Brain Res Brain Res Rev. 2005;50:1–6. doi: 10.1016/j.brainresrev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Bicker G. STOP and GO with NO: nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- 4.Madhusoodanan KS, Murad F. NO-cGMP signalling and regenerative medicine involving stem cells. Neurochem Res. 2007;32:681–694. doi: 10.1007/s11064-006-9167-y. [DOI] [PubMed] [Google Scholar]
- 5.Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jurado S, Sanchez-Prieto J, Torres M. Differential expression of NO-sensitive guanylyl cyclase subunits during the development of rat cerebellar granule cells: regulation via N-methyl-d-aspartate receptors. J Cell Sci. 2003;116:3165–3175. doi: 10.1242/jcs.00620. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Yoshida S, Yano M, Hanaoka F. Roles of endogenous nitric oxide in cerebellar cortical development in slice cultures. Neuroreport. 1994;5:2049–2052. doi: 10.1097/00001756-199410270-00015. [DOI] [PubMed] [Google Scholar]
- 8.Haase A, Bicker G. Nitric oxide and cyclic nucleotides are regulators of neuronal migration in an insect embryo. Development. 2003;130:3977–3987. doi: 10.1242/dev.00612. [DOI] [PubMed] [Google Scholar]
- 9.Peunova N, Scheinker V, Ravi K, Enikolopov G. Nitric oxide coordinates cell proliferation and cell movements during early development of Xenopus . Cell Cycle. 2007;6:3132–3144. doi: 10.4161/cc.6.24.5146. [DOI] [PubMed] [Google Scholar]
- 10.Knipp S, Bicker G. Regulation of enteric neuron migration by the gaseous messenger molecules CO and NO. Development. 2009;136:85–93. doi: 10.1242/dev.026716. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-López B, Noval JA, González-Bonet LG, Estrada C. Morphological bases for a role of nitric oxide in adult neurogenesis. Brain Res. 2000;869:244–250. doi: 10.1016/S0006-8993(00)02474-4. [DOI] [PubMed] [Google Scholar]
- 12.Gutièrrez-Mecinas M, Crespo C, Blasco-Ibáñez JM, Nácher J, Varea E, Martínez-Guijarro FJ. Migrating neuroblasts of the rostral migratory stream are putative targets for the action of nitric oxide. Eur J Neurosci. 2007;26:392–402. doi: 10.1111/j.1460-9568.2007.05672.x. [DOI] [PubMed] [Google Scholar]
- 13.Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 14.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 15.Judas M, Sestan N, Kostović I. Nitrinergic neurons in the developing and adult human telencephalon: transient and permanent patterns of expression in comparison to other mammals. Microsc Res Tech. 1999;45:401–419. doi: 10.1002/(SICI)1097-0029(19990615)45:6<401::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Foster JA, Phelps PE. Neurons expressing NADPH-diaphorase in the developing human spinal cord. J Comp Neurol. 2000;427:417–427. doi: 10.1002/1096-9861(20001120)427:3<417::AID-CNE8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Fertuzinhos S, Krsnik Z, Kawasawa YI, Rasin MR, Kwan KY, Chen JG, Judas M, Hayashi M, Sestan N. Selective depletion of molecularly defined cortical interneurons in human holoprosencephaly with severe striatal hypoplasia. Cereb Cortex. 2009;19:2196–21207. doi: 10.1093/cercor/bhp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritsche E, Cline JE, Nguyen NH, Scanlan TS, Abel J. Polychlorinated biphenyls disturb differentiation of normal human neural progenitor cells: clue for involvement of thyroid hormone receptors. Environ Health Perspect. 2005;113:871–876. doi: 10.1289/ehp.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moors M, Cline JE, Abel J, Fritsche E. ERK-dependent and independent pathways trigger human neural progenitor cell migration. Toxicol Appl Pharmacol. 2007;221:57–67. doi: 10.1016/j.taap.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E (2009) Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect 117:1131–1138 [DOI] [PMC free article] [PubMed]
- 21.Tegenge MA, Bicker G. Nitric oxide and cGMP signal transduction positively regulates the motility of human neuronal precursor (NT2) cells. J Neurochem. 2009;110:1828–1841. doi: 10.1111/j.1471-4159.2009.06279.x. [DOI] [PubMed] [Google Scholar]
- 22.De Vente J, Steinbusch HW, Schipper J. A new approach to immunocytochemistry of 3′, 5′′-cyclic guanosine monophosphate: preparation, specificity, and initial application of a new antiserum against formaldehyde-fixed 3′, 5′-cyclic guanosine monophosphate. Neuroscience. 1987;22:361–373. doi: 10.1016/0306-4522(87)90226-0. [DOI] [PubMed] [Google Scholar]
- 23.Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E. Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing. Environ Health Perspect. 2009;117:1131–1138. doi: 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 26.Brannen CL, Sugaya K. In vitro differentiation of multipotent human neural progenitors in serum-free medium. Neuroreport. 2000;11:1123–1128. doi: 10.1097/00001756-200004070-00042. [DOI] [PubMed] [Google Scholar]
- 27.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trimm KR, Rehder V. Nitric oxide acts as a slow-down and search signal in developing neurites. Eur J Neurosci. 2004;19:809–818. doi: 10.1111/j.0953-816X.2004.03182.x. [DOI] [PubMed] [Google Scholar]
- 29.Elferink JG, VanUffelen BE. The role of cyclic nucleotides in neutrophil migration. Gen Pharmacol. 1996;27:387–393. doi: 10.1016/0306-3623(95)00070-4. [DOI] [PubMed] [Google Scholar]
- 30.Borán MS, García A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem. 2007;102:216–230. doi: 10.1111/j.1471-4159.2007.04464.x. [DOI] [PubMed] [Google Scholar]
- 31.Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun. 2001;280:798–805. doi: 10.1006/bbrc.2000.4194. [DOI] [PubMed] [Google Scholar]
- 32.Gudi T, Chen JC, Casteel DE, Seasholtz TM, Boss GR, Pilz RB. cGMP-dependent protein kinase inhibits serum-response element-dependent transcription by inhibiting rho activation and functions. J Biol Chem. 2002;277:37382–37393. doi: 10.1074/jbc.M204491200. [DOI] [PubMed] [Google Scholar]
- 33.Sporbert A, Mertsch K, Smolenski A, Haseloff RF, Schönfelder G, Paul M, Ruth P, Walter U, Blasig IE. Phosphorylation of vasodilator-stimulated phosphoprotein: a consequence of nitric oxide- and cGMP-mediated signal transduction in brain capillary endothelial cells and astrocytes. Brain Res Mol Brain Res. 1999;67:258–266. doi: 10.1016/S0169-328X(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 34.Lindsay SL, Ramsey S, Aitchison M, Renné T, Evans TJ. Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J Cell Sci. 2007;120:3011–3021. doi: 10.1242/jcs.003061. [DOI] [PubMed] [Google Scholar]
- 35.Chen H, Levine YC, Golan DE, Michel T, Lin AJ. ANP-initiated cGMP pathways regulate VASP phosphorylation and angiogenesis in vascular endothelium. J Biol Chem. 2007;283:4439–4447. doi: 10.1074/jbc.M709439200. [DOI] [PubMed] [Google Scholar]
- 36.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-W. [DOI] [PubMed] [Google Scholar]
- 37.Brown C, Pan X, Hassid A. Nitric oxide and C-type atrial natriuretic peptide stimulate primary aortic smooth muscle cell migration via a cGMP-dependent mechanism: relationship to microfilament dissociation and altered cell morphology. Circ Res. 1999;84:655–667. doi: 10.1161/01.res.84.6.655. [DOI] [PubMed] [Google Scholar]
- 38.Ignarro LJ. Nitric oxide, biology and pathobiology. San Diego: Academic Press; 2000. pp. 300–380. [Google Scholar]
- 39.Gally JA, Montague PR, Reeke GN, Jr, Edelman GM. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci USA. 1996;87:3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson NJ, Rössler W, Nighorn AJ, Oland LA, Hildebrand JG, Tolbert LP. Neuron-glia communication via nitric oxide is essential in establishing antennal-lobe structure in Manduca sexta . Dev Biol. 2001;240:326–339. doi: 10.1006/dbio.2001.0463. [DOI] [PubMed] [Google Scholar]
- 41.Traister A, Abashidze S, Gold V, Plachta N, Karchovsky E, Patel K, Weil M. Evidence that nitric oxide regulates cell-cycle progression in the developing chick neuroepithelium. Dev Dyn. 2002;225:271–276. doi: 10.1002/dvdy.10164. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001;50:602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- 43.Cui X, Chen J, Zacharek A, Roberts C, Yang Y, Chopp M. Nitric oxide donor up-regulation of SDF1/CXCR4 and Ang1/Tie2 promotes neuroblast cell migration after stroke. J Neurosci Res. 2009;87:86–95. doi: 10.1002/jnr.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]